Seminar 3: Laboratory of molecular biotechnology – basic
advertisement

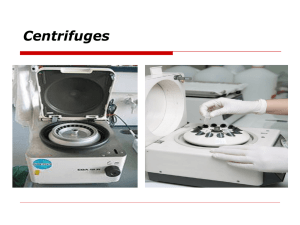
Seminar 3: Laboratory of molecular biotechnology – basic equipment and safe laboratory practices 1. Safety 1.1. General rules 1.1.1.All overcoats should be left in the cloakroom. It is not allowed to bring jackets or coats to seminar room. 1.1.2.Every student should use personal protective equipment which include: Laboratory coat Gloves Face mask Safety glasses Face shields or goggles 1.1.3.It is not allowed to eat, drink or smoke in the seminar room. Use of mobile phones is prohibited. 1.1.4.Work area should be kept in order (bags and clothes should not be put on bench). Bench surface should be clean. Use dedicated solutions to clean bench and equipment if spillage or contamination occurs. Always inform course leader about any accidents. 1.1.5.All consumables (plasticware) and biological materials should be disposed in dedicated waste containers 1.1.6.Upon completion of work working area should be cleaned and left in order. 1.1.7.Wash your hands with soap upon completion of work. 1.2. Hazards associated with laboratory work: Electricity Fire Radiation (UV, radioisotopes, electromagnetic, ultrasound) Temperature – liquid nitrogen, dry ice, low temperature freezer, thermoblock, waterbath, autoclave Mechanical hazard – centrifuge, gas containers, heavy equipment, sharp equipment (needles, scalpels) Solvents and reagents which may be toxic, corrosive, mutagenic (in contact, aerosols, dusts) Biological hazards: o Viruses o Bacteria o Fungi o Eucaryotic cell cultures (virus production) 1.3. Dangerous substances – every substance has label which contains the most important information about its chemical and physical properties and known and potential hazards Zakład Biotechnologii i Inżynierii Genetycznej METHODS IN MOLECULAR BIOTECHNOLOGY Every substance is supplied with Material Safety Data Sheet form (MSDS). This is a form containing information regarding the properties of a particular substance. The MSDS includes physical data such as melting point, boiling point, and flash point, information on the substance’s toxicity, reactivity, health effects, storage, and disposal, as well as recommended protective equipment and procedures for handling spills. 2. Aseptic technique – work with bacteria and cell cultures require special attention and appropriate activities to eliminate contamination of cultures and containment of laboratory or humans. There are 4 defined levels of containment corresponding to level of risk associated with handling a particular agent. Biosafety Level 1 (BSL-1) is the basic level of protection common to most research and clinical laboratories, and is appropriate for agents that are not known to cause disease in normal, healthy humans. (Escherichia coli) Biosafety Level 2 (BSL-2) is appropriate for moderate-risk agents known to cause human disease of varying severity by ingestion or through percutaneous or mucous membrane exposure. Most cell culture labs should be at least BSL2, but the exact requirements depend upon the cell line used and the type of work conducted. (Staphylococcus aureus) Biosafety Level 3 (BSL-3) is appropriate for indigenous or exotic agents with a known potential for aerosol transmission, and for agents that may cause serious and potentially lethal infections. (Mycobacterium tuberculosis) Biosafety Level 4 (BSL-4) is appropriate for exotic agents that pose a high individual risk of life-threatening disease by infectious aerosols and for which no treatment is available. These agents are restricted to high containment laboratories. (Ebola virus) General procedures to be followed in class I microbiology laboratory 1. All doors and windows should be closed during work 2. Prior to work bench and gloves should be decontaminated with dedicated solution (disinfectant, 70% ethanol) 3. Bottles, dishes, pipette tips, pipettes and inoculating loops should be flamed before use 4. It is not allowed to talk, laugh and cough during work to prevent contamination of cultures 5. Bacterial cultures or materials which had contact with them should be deactivated properly by either autoclaving or soaking them in 10% bleach for a few days. Dedicated waste containers should be used for further disposal after deactivation 6. After completing work bench should be decontaminated with dedicated solution (disinfectant, 70% ethanol) Zakład Biotechnologii i Inżynierii Genetycznej METHODS IN MOLECULAR BIOTECHNOLOGY 3. Basic equipment 3.1. Pipettes, pipettors and tips 3.1.1.Remember to fit proper tip to a pipette ! 3.1.2.Never set the volume outside the pipette volume range ! 3.2. Plasticware: 3.2.1.Tubes: PCR tubes, eppendorfs (eppis), falcons (bluecaps), vials 3.2.2.Dishes: bacterial dishes (Petri dishes), cell culture dishes 3.2.3.Racks, boxes 3.3. Glassware: 3.3.1.Bottles, Erlenmeyer flasks, cylinders, beakers (also made of plastic) 3.4. Centrifuges: refrigerated and ambient, fixed angle and swing bucket. Important things to remember while working with fuge: 3.4.1.Use only rotors designed for specific centrifuge (select type of rotor in operation panel after mounting the rotor) 3.4.2.Inspect rotor for damages, tube leftovers, spills 3.4.3.Load rotor symmetrically – to balance the rotor fill all opposing tubes (of the same type) to the same level with liquid of the same density 3.4.4.Close lid tightly 3.4.5.Never try to open the lid while centrifuge is spinning 3.4.6.Always empty the rotor and clean it gently if it is necessary 3.4.7.Close the lid 3.5. Laminar flow hood 3.6. Incubators – water jacketed, air jacketed 3.7. Thermoblocks Zakład Biotechnologii i Inżynierii Genetycznej METHODS IN MOLECULAR BIOTECHNOLOGY