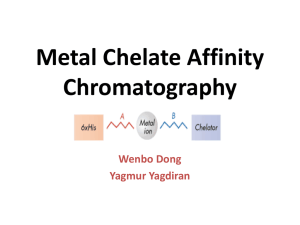
INTRODUCTION
advertisement

Chap 9 Downstream Processing Introduction Definition: the isolation and purification of biotechnological product to a form suitable for its intended use. Culture is harvested at the optimal timing cells are separated from the mediumdownstream processing. Biotechnological products include: whole cells, organic acids, amino acids, solvents, antibiotics, industrial enzymes, therapeutic proteins, vaccines, etc. The complexity of the processing steps is determined by the required purity, which in turn, is determined by its application. Separation principles also depend on the size and nature of products. For intracellular products==> disrupting cells ==> following purification steps For extracellular products==>concentrating the medium ==> purification Imperative to minimize the number of steps and maintain high yields in the different stages. Many steps are unit operation processes, usually divided into four stages: Solid-liquid separation or clarification Concentration Purification Formulation I. Solid-liquid separation Refers to the separation of cells from the culture broth, removal of cell debris, collection of protein precipitate, etc. The unit operations involved are normally centrifugation and filtration. (note: cell debris: broken cells, could be generated by cell disruption) 1 Filtration The mixture goes through a filter which retains the particles according to size while allows the passage of fluid through the filter. In cake filtration, the particles are retained as a cake on the filter, resulting in the resistance. In many cases, the cakes are compressible and the changing effective pressure difference influences the flow through the filter. Filtration theory Rate of filtration is often measured as the rate at which liquid filtrate is collected. 1 dV f A dt p M f [ ( c ) rm ] A Vf: filtrate volume; t: filtration time; dVf/dt: volumetric rate of filtration; A: filter area; p: pressure drop across the filter; f: filtrate viscosity Mc: total mass of solids in the cake; : average specific cake resistance [LM-1], a measure of the resistance of the filter cake to flow, dependent on shape and size of the particles. rm: resistance from the filter [L-1], includes the effect of filter material and any particles wedged in it during the initial stages of filtration Let M c cV f , where c: mass of solids deposited per volume of filtrate. Substitute this into the above equation 2 1 dV f A dt f [ ( p cV f A ) rm ] Assume p is constant (most commonly carried out) and take the reciprocal A Vf f rm dt f c dV f Ap p Integration yields At ( f c 2 Ap )V f2 ( f rm p )V f Vf or t can be calculated at constant pressure, provided all the constants are known. Examples of filter are perforated sintered metal, cloth, synthetic fibers, cellulose, etc. Vacuum filters are also often used due to the simplicity of operation and low costs==>Rotary drum filtration (often used for bacteria, filamentous fungi and yeast cells) Pretreatment of broth Pretreatment by changing the biomass particle size, the broth viscosity and the interactions between the biomass particles can facilitate clarification (e.g. filtration). Filter aid: incompressible discrete particles of high permeability with size ranging from 2-20 m increase the porosity of the cake and facilitate the passage of the liquid. It should be inert to the broth, the most frequently used is Diatomite (skeletal remains of aquatic plants) or inactive carbon. Flocculating agents: can help the agglomeration of individual cells or cell-particles into large flocs which can be easily separated at low centrifugal forces. These agents include inorganic salts or polycations, either cellulosic or synthetic polymers. 3 Flotation: Particles are adsorbed on gas bubbles, get trapped in a foam layer and can be collected. The gas may either be sparged into the particulate feed or very fine bubbles can be generated from dissolved gases by releasing the overpressure. Formation of stable foam is supported by the presence of long chain fatty acids. Centrifugation Separation by means of the accelerated gravitational force achieved by a rapid rotation. Relies on the density difference between the particles and the surrounding medium, most effective when the particles to be separated are large, the liquid viscosity is low and the density difference between particles and fluid is great. Batch centrifuge is common in the labs but the low processing capacity limits its use in large scale. 4 Continuous centrifuges are common in large-scale processing in which the deposited solids are removed continuously or intermittently. Tubular bowl centrifuge Very commonly used in food and pharmaceutical industries. Feed enters under pressure through a nozzle at the bottom, and moves upwards through the cylindrical bowl. As the bowl rotates, particles traveling upward are spun out and collide with the walls of the bowl. Solids hitting the wall can form the cake. As the feed rate increases, the liquid layer moving up the wall becomes thicker thus reducing the performance of the centrifuge by increasing the distance a particle must travel to reach the bowl. This system lacks a provision of solids rejection, the solids can only be removed by stopping the machine, dismantling it and scraping or flushing the solids out manually. 2 rpm Typical range of centrifugal force: 13000-16000g (g: r (mm) 1.12 ) 1000 Disc-stack bowl centrifuge: 5 Contain conical sheets of metal (discs) which are stacked with clearances as small as 0.3 mm. The discs rotate with the bowl to split the liquid into thin layers. Feed is released near the bottom of the centrifuge and travels upwards through matching holes in the discs. Between the discs, heavy components of the feed are thrown outward under the influence of centrifugal forces as lighter liquid is displaced towards the center of the bowl. As they are flung out, the solids strike the undersides of the discs and slide down to the bottom edge of the bowl. At the same time, the lighter liquid flows in and over the upper surfaces of the discs to be discharged from the top of the bowl. Disc-stack bowl centrifuge with continuous discharge of solids Disc-Stack bowl centrifuge 6 Heavier liquid containing solids can be discharged either at the top of the centrifuge or through nozzles around the periphery of the bowl. Typical range of centrifugal force: 5000-15000g Note: the supernatant obtained by centrifugation is not free of cells (103 to 105 cells/ml) and costs of maintenance and power consumption are both high. Separation of particulate debris is inefficient by centrifugation. II. Release of intracellular components To release maximum amount of the product in an active state. Factors to consider: inactivating effects such as shear, temperature and proteases, choice of disruption methods, subsequent processing steps. Disruption of microbial cells Mechanical disruption is the most common means to release intracellular products in laboratory and in industry. Ultrasonication is common in the lab-scale but the removal of heat is difficult on a larger scale. Two common industrial processes: High pressure homogenization: the cell suspension is forced at high pressure through an orifice to emerge at atmospheric pressure. 7 Vigorous agitation with abrasives: agitation with glass in bead mills ruptures the cells by high shear and impact with the cells. Size of beads: 0.2-0.5 mm for bacteria; 0.4-0.7 mm for yeasts. Non-mechanical disruption freeze/thaw: disrupt the cells by causing changes in the structure of the cell wall and membrane. Organic solvents and detergents: Enzyme: is mild and has selectivity during the product release. Gram+ (no outer membrane) bacteria are more susceptible while the lysis of Gram- (with outer membrane) bacteria requires the passage of lysozyme through the outer membrane, which can be aided by the addition of EDTA. Glucanase and mannase, in combination with proteases, are used for the degradation of yeast cell walls. Availability and costs limit the use of enzymatic methods. A combination of enzymatic/chemical lysis with mechanical methods could be used. Homogenization of animal/plant tissue: Animal tissues: cut into small pieces, suspend in ice-cold homogenization buffer and grind in a blender. Animal cells are usually easier to break because of the absence of cell walls. Plant cells have tough cell walls and are more resistant. Non-fibrous plant tissue: more easily to be macerated (to become soft by putting or being left in water), rapid homogenization of the material in the buffer using a blender. Fibrous material: difficult to macerate==> Buffers with pH around 6.5-7.2 are used to neutralize the acidic materials including the phenols. Buffers also contain reducing agents such as ascorbate and thiols to prevent the accumulation of quiones and hence the inactivation of enzymes during extraction. 8 Use of cellulase and pectinase (pectin: gel-like stuff in plants, derived from galacturonic acid) for digestion of plant cell walls is an attractive alternative for achieving selective release of the protoplasmic material (but not the vacuole contents). III. Concentration of biological products After initial separation, the filtrate contains 85-90% of water. Removal of water can be done in different ways: Evaporation Simple but energy-consuming, normally using steam as the heat source in a large scale. Applicable for food proteins and other stable biologics, but seldom suitable for processing of biologically active proteins. Falling film evaporators: the liquid to be concentrated flows down long tubes/plates and distributes uniformly over the heating surface as a thin film. Part of the liquid is evaporized and exits as the vapor. Suited for viscous products and is often used in fermentation industry. Liquid-liquid extraction Applied on a large scale in biotechnology both for concentration and for purification. Efficiency depends on the distribution of substances between two phases, 9 defined by: partition coefficient K (concen. in extract phase/concen. in raffinate phase) Extraction of low-MW products (vitamins, antibiotics, 2-propanol, butanol, caffeine.) Small lipophillic molecules can be extracted by organic solvents (but it may be more difficult to design an extraction process for hydrophilic molecules). Physical extraction: distribution is based on the physical preference. This applies to nonionizing compounds and the extraction is optimized by screening for the solvents that have a high K value. Dissociative extraction: distribution is based on Reactive extraction: for compounds with Carriers (e.g. phosphorous compound) are added to the organic solvent to form selective solvation bonds or complexes that are insoluble in the aqueous phase=> the compound is carried from the aqueous phase to the organic phase. After extraction, the product can be recovered from the solvent by distillation. If the product is heat sensitive back-extraction into a new aqueous phase. Ex: penicillin is extracted into butyl acetate from the fermentation medium at pH 2.5-3, and back-extracted into aqueous phosphate buffer at pH 5-7.5. Supercritical fluid (SCF) extraction: SCF are fluids above their critical temperature and pressure, with 10 Usually, the compressed SCF is contacted with the feedstock to be extracted in an extraction column, which is then transferred via an expansion valve to a separator. On lowering the pressure, the fluid turns into gas and releases the product as a precipitate. Extraction of proteins Aqueous two-phase systems (ATPS) PEG phase containing soluble proteins Dextran phase containing cellular debris For industrial processes, polyethylene glycol (PEG)/salt system is often used for their low cost. 11 Partitioning of a component is based on its surface characteristics, nature of phase components and the ionic composition. Phase separation is slow (from minutes to hours) but can be speeded up by centrifugation. Advantages: Membrane filtration Classification: Microfiltration: separates particles of Ultrafiltration: separates polymeric particles of Reverse osmosis (hyperfiltration): separates particles of 12 Reverse Osmosis 0.0001 μm Ultrafiltration Microfiltration 0.001 μm 0.01 μm 0.1 μm 0.2 kDa 200 kDa 20,000 kDa 1 μm Clarification 10 μm 100 μm Proteins Salts Mammalian virus Yeast Adapted from MS thesis, G.Y. Chen, NTHU. Selectivity of membranes, expressed as molecular weight cut-off (MWCO) for ultrafiltration membranes, is mainly determined by the Example: the actual molecular mass of albumin is 64 kD, the apparent size of albumin can increase due to a large “ionic cloud” forming around the molecule in a low ionic strength soln’, so the protein can behave like a 300 kD molecule and be subject to full retention when processed with a 100 kD rated membrane. 13 The MWCO is a nominal size, thus usually we select a membrane cut-off rating which is 0.2-0.3 times the size of the MW target for retention. e.g. to ensure retention of a 50 kD molecule, a 10-15 kD membrane should be used. Dead-end filtration: The direction of flow is perpendicular to the membrane surface. Deposition of particles and precipitation of small solutes, etc. on the surface can clog the membrane, hence reducing the flow rate. Cross-flow (tangential flow) filtration: 1. Stirred Cell (Millipore): Stir to avoid membrane clogging. Water, salts and lower MW molecules pass through, while larger molecules are retained concentration. Because the pore size is small, pressure can be applied to speed up the concentration. 14 2. Hollow-fiber: comprises a bundle of hollow capillaries packed in the tube. Source: “Protein concentration and diafiltration by tangential flow filtration”. Millipore Corporation. Tangential flow filters can serve two purposes: Membrane adsorbers: Membranes with ion exchange groups or affinity ligands which bind proteins from the clarified feed. Desorption is carried out by appropriate buffers. A stack of membranes provides large surface area for adsorption (rending it similar to chromatography gels). Precipitation 15 Protein Solubility of proteins changes with salt concentration which can be expressed in terms of ionic strength: 1 Salt in I C i Z i2 Salt out 2 Zi: ionic charge Ci: molar concentration of the ionic species (can Salt be neutral salts, organic solvents or high MW polymers). salt in: when ion concentrations increase, the additional counter ions salt out: at high salt concentration, Table 9.4 Modes of protein precipitation Mode Example Addition of neutral salt (NH4)2SO4 Comments Addition of organic solvent Acetone, ethanol Increased hydrophobic interactions between neutral protein molecules salt is removed prior to next purification step (except for hydrophobic interaction chromatography) by dialysis, UF or gel filtration Reduced dielectric constant enhances electrostatic interactions between protein molecules (low dielectric const. increases the charge-dipole and dipole-dipole attraction between proteins and increases the precipitation) Addition of non-ionic polymer PEG Addition of charged polymer Polyethylen eimine Polyacrylic acid Change in pH Low temperature required for operation (for safety) Reduction in the effective quantity of water available for protein solvation Polymer often has stabilizing effect on proteins Complex formation between oppositely charged molecules leads to charge neutralization and precipitation Low solubility of protein at isoelectric point (the pH at which the protein has no net charge) Extremes of pH denature and precipitate sensitive proteins All the precipitates can be re-dissolved in small volume of buffer suitable for the next step. 16 Adsorption to chromatographic resins (see Chromatography) Purification by Chromatography IV. Intro: The degree of purification in previous steps is limited, usually need several chromatography steps to yield high purity. Which chromatography to use depends on the characteristics of the proteins, such as size and shape, overall charge, surface hydrophobic groups, and ability to bind various ligands. Gels (resins) are usually made of cross-linked polymers: Agarose: polysaccharide made up of D-galactose and 3,6-anhydro-1-galactose units Cellulose: polysaccharide of -1-4 linked glucose units Dextran: a polysaccharide of -1-6-linked glucose Polyacrylamide: polymer of acrylamide and bis-acrylamide e.g. Sephadex (Amersham Pharmacia, now part of GE Healthcare) 1. Purification usually accounts for 50-70% of production cost mostly on Size Exclusion Chromatography (SEC) 17 Protein content monitored by 2. Ion Exchange Chromatography (very often used) a.a. exhibit different charges. The net charges of proteins depend on the The pH at which a protein has no net charge is called isoelectric point (pI) At pI, the proteins do not repel one another and thus can precipitate. ion exchange chromatography is based on the Charged groups are immobilized to solid matrix (gel) Positively (Negatively) charged proteins bind to negatively (positively) charged groups by displacing the H+ (OH-) which is initially bound to the resin. e.g. + groups: diethylaminoethyl -O-(CH2)2N+H(CH2CH3)2 (also known as DEAE) trimethylamino methyl CH2N+(CH3)3 (also known as Q) – groups: carboxymethyl (CH2COO-), sulphomethyl (CH2SO3-) 18 After binding, the column is washed several times with wash buffer to remove non-specifically bound proteins. After wash, the bound proteins are eluted using the elution buffer. For elution, a salt containing buffer (often NaCl) of increasing ionic strength in turn displaces the protein from the matrix. Summary: General procedures: sample loadingwasheselution. In each step, the samples are collected and can be analyzed for purity and recovery. 3. Popular: Hydrophobic Interaction Chromatography (HIC) HI results from water’s propensity to repel hydrophobic groups. HI is relatively weak compared to H-bonds and lacks directionality. 8 a.a. are hydrophobic (non-polar): Proteins are folded partly by hydrophobic interaction for which the hydrophobic residues are buried inside (shielded from aqueous environment), and stabilize the protein conformation. However, a minority of hydrophobic a.a. are present on the surface and they tend to cluster to form a group. These hydrophobic groups are masked by an ordered film of water molecules. HIC (also known as reverse phase chromatography) uses the different degrees of surface hydrophobicity and achieves resolution by thousands of interactions of solute molecules with the resin. HIC has high resolving power and is a widely used analytical chromatography. Hydrophobic groups such as phenyl group or octyl group are immobilized to the gel. 19 OH OH Sepharose-O-CH2-CH-CH2-O- Sepharose-O-CH2-CH-CH2-O-(CH2)7-CH3 Octyl group Phenyl group Samples are loaded into the column and proteins bind to the gel, the more hydrophobic the protein is, the tighter the protein binds. Salt (e.g. NaCl, or ammonium sulfate) is added in the sample to increase the ionic strength, Elution: 4. Affinity Chromatography Utilize the affinity of the protein toward the ligands. The binding can be achieved via the affinity between the protein and the ligand immobilized on the resin. Most powerful and highly selective. Categories of affinity interactions a. Protein A-IgG1 for the purification of monoclonal antibodies (MAb) b. Immunoaffinity: Exploits the affinity interactions between Ab and Ag. Hundreds of new protein products are currently under clinical investigation or are awaiting the FDA approval. MAb constitutes the single largest category (>200 MAb). 20 The interactions include electrostatic interactions, H-bond, van der Waals forces and hydrophobic forces. Ab is immobilized to the resin so as to bind the Ag (the target protein) in the sample. This process can achieve Drawbacks: One popular method uses a glycine-HCl buffer at pH 2.2-2.8 (resulting in partial denaturation) for elution. High salt concentration or extremes of pH disrupt Ag-Ab interactions by decreasing electrostatic interactions and/or Hbonds. c. Lectin-glycoprotein (for the purification of glycoproteins) glycoproteins: proteins with carbohydrate side chains (e.g. hormones, growth factors) lectins: a group of proteins that bind carbohydrate molecules, e.g. d. Binding: Elution: Drawback: Ni-Histidine (popular in recent years) Relies on genetically added 6 histidine residues on proteins either at the N- or C- terminus. Divalent cations (e.g. Ni2+, Cu2+ or Zn2+) are immobilized on resins and bind the proteins with His6 tag. e. Elution is performed by the competition of imidazole (an analogue of histidine). Others Summary for protein purification by chromatography: 21 General procedures (except size exclusion): sample loadingwasheselution. In each step, the samples are collected and can be analyzed for purity and recovery. The wash and elution buffers have different compositions. Initially, the desired proteins can be washed and eluted using linear gradient of eluting agents. After identifying the optimal eluting agent concentrations, stepwise wash/elution can be carried out. Two important factors to consider: Top: process block diagram for the purification of bovine growth hormone (somatotrophin) produced in E. coli. (intracellular product) Bottom: purification summary for processing 260 Kg of inclusion bodies. (Adapted from Blanch, HW, Clark, High flow rates are desired in order to save time (several hours) Fast-flow Protein Liquid Chromatography (FPLC, e.g. AKTATM by Amersham Pharmacia) 22 using rigid gels and stainless steel column to withstand high pressurehigh flow rates fast (10 min to 1 hr) V. Protein Stabilization on Finished Product Denaturation of proteins and loss of biological activity are problems. Factors resulting in denaturation and loss of activity Chemical Detergent (unfold the natural conformation), e.g. SDS Urea Guanidine hydrochloride Solvents (interact with hydrophobic a.a.) Heavy metals (interact with –SH groups) Physical Extremes of pH High temperature (exceptions: proteins in bacteria in hot spring) Freeze and thaw (freezing causes changes in microenvironment and local pH minimized by rapid freezing Vigorous agitation Biological Protease (can add protease inhibitors, such as aprotinin, PMSF…) Stabilization: High protein purity may decrease the stability Proteins still lose activity during storage add agents to prolong the shelf life glycols: glycerol, polyethylene glycol sugars: sucrose neutral salts: ammonium sulfate, NaCl Proper storage: Long-term: spray drying or lyophilization. In spraying, the liquid input stream is sprayed through a nozzle into a hot vapor stream and vaporized. The solvent in 23 the small liquid droplets is quickly vaporized. Lyophilization Reference: 1. 2. 3. Walsh, G. (2002) Proteins: biochemistry and biotechnology. John Wiley & Sons. New York. Doran, PM (2003) Bioprocess Engineering Principles. Academic Press. San Diego. Blanch, HW, Clark, DS. (1997) Biochemical Engineering. Marcel Dekker. New York. 24