Protein Purification Strategies
advertisement

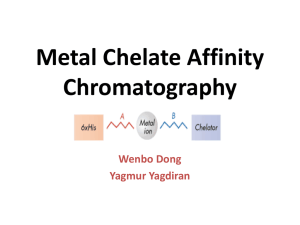
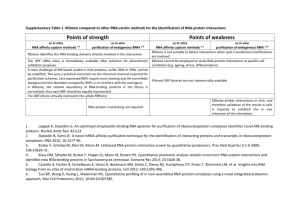
Protein Purification Strategies Course: Methods in protein chemistry Rahman M. Mahfuzur 2012/01/11 SLU Objectives To seperate a particular protin from all other proteins and cell components There are many types of proteins within an ogranism Other components: nuclic acids, charbohydrates, lopids, small molecules A gieven protein could be 0.001-20% of total protein. Protein purification Proteins purification varies from one purification step to multi- step of purifixcations Often more than one purification step is necessary to reach the desired purity, or step can be repetead if sample is available. Successful and efficient protein purification depends on appropriate methods selection. Methods should be sequence in a logical manner, what kinds of materials are available/handle? what has to be removed/ completely? what will be the use of final products? what are economical constraints? Three phase purification strategies(CIPP) The four parameters Every technique offers a balance between resolution, capacity, speed and recovery Capture Initial purification of target Rapid isolation, stabilization, concentration Intermediate purification Further removal of bulk contaminants: other proteins, nucleic acids, endotoxins and viruses. Purification and concentration. Polishing Final removal of remaining trace impurities or closely related substances to achieve high purity Protein properties Vs Technique Protein property Technique Charge Ion exchange (IEX) Specific ligand recognition (biospecifc or nonbiospecifc) Affinity chromatography (AC) Size Gel filtration (GF) Hydrophobicity Hydrophobic interaction (HIC), Reversed phase (RPC) Isoelectric point Chromatofocusing Ion exchange chromatography (IEX) separates proteins with differences in surface charge. based on the reversible interaction, charged protein Vs oppositely charged column If, pHb>PIP Neg. bind positively charged anion exchanger ; ex- MonoQ column When, pHb<PIP Pos. bind negatively charged cation exchanger ; ex- MonoS column IEX chromatogram Affinity chromatography (AC) On the basis of a reversible or specific interaction between target and a specifc ligand Biospecific: antibodies binding proteins, Non-biospecific: histidine binding protein bind to metal Ion; IMAC AC chromatogram Gel filtration chromatography (GF) allow separation of proteins with differences in molecular size GF is a non-binding method 0.5% to 2% of total column volume. FG chromatogram Hydrophobic interaction chromatography (HIC) separates proteins with differences in hydrophobicity based on the reversible interaction between a protein and the hydrophobic surface of a chromatography medium Technique Vs three phase combinations of chromatographic steps IEX-HIC-GF Considered as a standard protocol If nothing is known about the target protein use IEXHIC-GF. both anion and cation exchange could be used to get different selectivities within the strategy. Thanks for listening me!