CE 428 Design of Water and Wastewater Treatment
advertisement

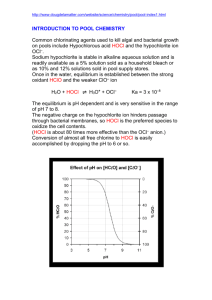
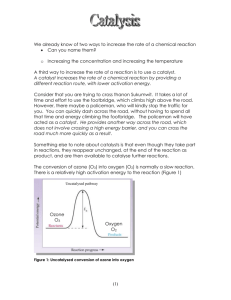
CE 428 Design of Water and Wastewater Treatment Oxidizing Agents and Disinfectants Table 1 Typical Oxidizing Agents and Their Relative Oxidizing Power Redox reaction Oxidizing E˚ (V) Agent at 25˚ C Relative Oxidative Power _________________________________________________________________________ F2 + 2e- = 2FF2 3.06 2.55 O3 + 2H+ + 2e- = H2O O3 2.07 1.52 H2O2 + 2H+ + 2e- = H3O2+ H2 O2 1.76 1.30 MnO4- + 4H+ + 3e- = MnO2 + 2H2O MnO41.68 1.24 + HOCl + H + 2e = Cl + H2O HOCl 1.49 1.10 Cl2 + 2e- = 2Cl Cl2 1.36 1.00 I2 + 2e = 2I I2 0.54 0.40 __________________________________________________________________________ CHLORINE • mostly used as a disinfectant in US. Chlorine disinfects by altering the permeability of a cell and interfering with the exchange between the cell and its environment or causing leakage of the cytoplasm and damage to the nucleic acid. Chlorine form hypochlorous acid (HOCl) and hydrochloric acid (HCl) in water and most of the disinfecting power of chlorine is from the hypochlorous acid (HOCl). • Advantages of chlorine include cost effectiveness, persists in water, good understanding of chemistry and design of application of chlorine, high potent against a wide range of pathogens. • Disadvantages are that chlorine is toxic to marine organisms, may form chlorinated compounds that may be more toxic than the original compound, eg., trihalomethanes (chloroform and bromoform), poisonous gas OZONE • is an allotropic form of oxygen. Ozone is generated from air or pure oxygen when a high voltage is applied across the gap of a narrowly-spaced electrode. The high energy corona dissociates one oxygen molecule into two atomic oxygen which then combine with two other oxygen molecules to form two ozone molecules. The gas stream generated from air by this process will contain about 0.2 to 3 % ozone by weight and from pure oxygen is approximately 1 to 6 %. 3O2 <==> 2O3 and • • • • • O2 + O<==> O3 Use of ozone is popular in Europe while in the US it is a recent occurrence. Ozone deactivates bacteria by reacting with the saturated fatty acids of phospholids and glycoproteins in the cell membranes resulting in leakage or lysis of the cells ozone decomposes rapidly in aqueous solutions due to impurities such as organic compounds or particulates Half lives for Ozone is approximately 18 minutes in ground water, 10 minutes in lake water. Advantages : lack of persistent toxic residuals, increase in effluent dissolved oxygen, very strong disinfectant, relatively insensitive to pH in the range of 6.0 to 10.0 and temperatures in the range of 2 to 30 o C. Disadvantages: high capital and operating cost, lack of residual, mass transfer of ozone from air phase to aqueous phase is a limiting factor CHLORINE DIOXIDE • ClO2 is a greenish-yellow gas and was first prepared by Davy in 1811 by combining potassium chlorate and hydrochloric acid. Chlorine dioxide gas is unstable and is usually generated on site. Generation of chlorine dioxide involves the reaction of chlorine and sodium chlorite. Yield of a typical generator is 80 – 90%. Cl2 + 2 NaClO2 == > 2 ClO2 + 2 NaCl • • • Usage in the US for disinfection is limited but can be found in pulp and paper industry for bleaching of wood pulp. Advantages: do not produce significant quantities of chlorinated organic byproducts, effective over a broad pH range, do not react with ammonia to form chloramines Disadvantages: cost of precursor chemical, sodium chlorite, formation of chlorates (ClO3-), ClO2 gas is explosive UV RADIATION • Does not chemically alter the water • Bacteria inactivation by UV is from the attack of nucleic acids. • UV wavelength that causes the most cellular damage is between 250 to 270 nm. The absorption spectrum of DNA and RNA shows peak absorption at wavelengths between 25 and 265 nm. Low pressure mercury arc lamp has approximately 85% of its light output at 253.7 nm. • Advantages: no residual toxicity, effective on a variety of microorganisms, requires little space • Disadvantages: lack of measurable residual, high maintenance needed and replacement of lamp, turbidity affects effectiveness, possible occurrence of photoactivation (i.e., injured organisms when exposed to light energy at wavelengths between 310 and 500 nm may be restored and replicate normally). CHLORINE CHEMISTRY Forms hypochlorite and hypochlorous acid Cl2 + H2O <==> HOCl + H+ + ClHOCl <==> OCl- + H+ Ka = [H+][OCl-] / [HOCl] Ka pKa at 20o C = 1.5 x 10-8 at 0o C = 2.62 x 10-8 at 20o C = 7.5 HOCl is about 80 - 200 times more effective than OCl- (see Figure) With the addition of chlorine in water, the following reactions occur if the following substances are present: a. Reducing agents such as S2-, HS-, Fe2+, Mn2+, NO2Chlorine will oxidize them to the oxidized compounds Eg., S2- = > SO42Fe2+ = > Fe3+ b. Organic matter - will react on a variety of ways, oxidization to CO2 and to form chloro-organic compounds such as trihalomethanes – chloroforms, c. Ammonia – will react to form chloramines NH3 + HOCl => NH2Cl + H2O monochloramine NH2Cl + HOCl =>NHCl2 + H2O dichloramine NHCl2 + HOCl => NCl3 + H2O trichloramine 2NH3 + 3HOCl =>N2(g) + 3HCl + 3H2O Chloramines are called combined residual chlorine and have disinfecting power (see Figure 7-14: breakpoint chlorination, free residual, , etc) Design – Disinfection Practices in Water Treatment • specified in the Surface Water Treatment Rule (SWTR), require to inactivate 99.9% of Giardia Cysts and 99.99% of enteric viruses • The amount of disinfection needed is determined by the CT concept • CT is defined as the product of concentration (mg/L) and contact time (min) This is sometimes termed as the Chick-Watson Law. Chick first proposed that microorganisms die off in a first order manner dN/dt = - k N N = no. of microorganisms at time t t = time k = die off coefficient given by k' Cn where C is the concentration and n = constant Therefore (equation 12-5) dN/dt = k' Cn N or ln (N/No) = k' Cn t where No is the initial number of microorganisms • The SWTR Guidance Manual provides tables of CT values for different temperatures and concentrations (see Table 16.2) - must satisfy peak flow condition - Usually use a plug flow basin (see Figure 16.3) - Concentration is the concentration in the effluent (i.e., conservative estimate) - Finish water usually have a free residual chlorine of 1 mg/L - maintain a concentration of 0.2 to 0.5 mg/L of free residual chlorine in water distribution system - Ten States Standard – contact time – allow 2 hours for surface water for free residual chlorine, if combined residual chlorination – 3 hours.