chem-440-water treatment
advertisement

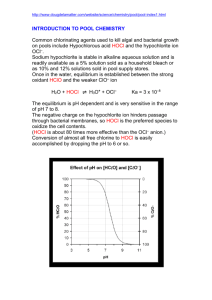
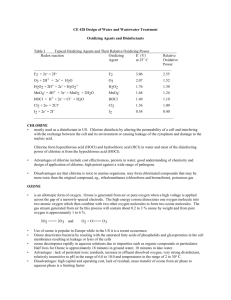
Waste water treatment Chem442,4402015 Primary treatment At the first step in primary treatment the sewage is passed through a screen to remove large pieces of debris such as sticks, stones, rags and plastic bags that have washed in through storm drain The sewage then enters a grit chamber, where the flow rate is slowed just enough to allow coarse sand and gravel to settle on the bottom .The grit is collected and disposed of in landfills. As water enters the sedimentation tank, its flow rate is further decreased to permit suspended solids –which account for approximately 30% of the total organic wastes – to settle as raw sludge. Any oily material floats to the surface and is skimmed off. Calcium hydroxide and aluminum sulfate are often added to speed up the sedimentation process. These two chemicals react to produce a gelatinous precipitate of aluminum hydroxide, which settles out slowly, carrying suspended material and bacteria with it. 3 Ca(OH)2 + Al2(SO4)3 2 Al(OH3) + 3 CaSO4 In the past, the raw sludge that settled out was incinerated, put in landfills, or dumped at sea. Now, much of it is composted, or otherwise treated, to produce a product known as biosolids, a nutrient-rich, bacteria-free, humus-like material that is used as fertilizer. There is however, concern that primary treatment does not remove toxic metals and non-biodegradable hazardous chemicals from this material. These pollutants get into sewage if metal-containing products, pesticides, and other dangerous compounds are carelessly flushed down drains. Secondary Treatment Secondary treatment is a biological process that relies on aerobic bacteria and other detritus feeders to break down nearly all the oxygen-consuming wastes remaining in the water after primary treatment. In the most frequently used method, a mixture of organisms termed activated sludge – is added to the sewage effluent as it moves slowly through a tank.in this oxygen-rich environment, the organisms digest the organic material and outflow down into carbon dioxide and water. After settling in sedimentation tank, the organisms, together with any remaining under composed material, are returned to the aeration tank. Most municipal sewage treatment plants chlorinate the water after secondary treatment and then release it into waterways. Although approximately 90% of the original organic material is removed by primary and secondary treatment, many other substances (including phosphates) are much less completely removed. Also, disinfection with chlorine can introduce hazardous chemicals. Disinfection with Chlorine: Chlorine gas (Cl2) reacts with water to form Hypochlorous acid (HClO) , which is a powerful oxidizing agent. Cl2 + H2O HClO + H+ + Cl- Waste water treatment Chem442,4402015 Hypochlorous acid kills disease-causing bacteria, destroys some (but not all) viruses and removes color from the water. Disinfection with chlorine gas is relatively cheap and has almost completely eliminated the risk of contracting water-borne diseases from municipal water supplies. Chlorine can cause a number of environmental problems, however. It is very toxic, and there is always the danger of accidental release from tanks during transportation or at the plant. Furthermore , low concentrations of chlorine reacts with residual organic and inorganic substances in the water to form by-products known as disinfectant byproducts (DBPs) , which include chloroform (CHCl3) , bromodichloromtethane (CHBrCl2) , dibromochloromethane (CHBr2Cl, and bromo form (CHBr3) , which are suspected of causing miscarriages, birth defects, and cancer. The bromine –containing compounds are produced as a result of the interaction of chlorine with bromine ions present in the water .the dangers associated with DBPs are minimized if tertiary treatment is used to remove organic substances . Alternatives to Chlorine that are sometimes used for disinfection include chloramines, chlorine dioxide, and ozone and UV radiations. Initially chloramines were used for taste and odor control ; however ,chloramines were soon organizes as being more stable than free chlorine , and were consequently found to be effective for controlling bacteria in the biolayer on insides of the water pipes. As a result, chloramines were used regularly during 1930s and 1940s for disinfection. As a result of an ammonia shortage during World War II, however the popularity of the chloramination declined. Recent concern over chlorinated organics has renewed interest in chloramines because they form very few disinfection byproducts (DBPs). Chloramines are formed from the reaction of aqueous (i.e HOCl) chlorine with ammonia. The mixture that results may contain monochloramine (NH2Cl), dichloramine (NHCl2), or trichloramine (NCl3). When chlorine is dispersed in water , a rapid hydrolysis occurs according to the following reaction: Cl2 + H2O HOCl + H+ + ClThe equilibrium constant (K eq) at 25 C is 3.94 * 10 4 for this reaction. In dilute solutions at pH greater than3, the forward reaction is essentially complete. Hypochlorous acid (HOCl) is a weak acid that dissociates as follows HOCl OCl- + H= pKa = 7.6 Relative proportions of HOCL and OCl- are dependent upon pH. Both of the chlorine species in the above reaction are powerful oxidants, capable of reacting with many substances present in water. In aqueous solutions with PH 7.0 to 8.5, HOCl reacts rapidly with ammonia to form inorganic chloramines in a series of competing reactions. The simplified stoichiometry of chlorine-ammonia reactions are as follows: NH3 + HOCl NH2Cl(monochloramine) + H2O NH2Cl + HOCl NHCl2(dichloramine) + H2O Waste water treatment Chem442,4402015 NHCl2 + HOCl NCl3 (trichloramine) + H2O These competing reactions, and several others, are primarily dependent on pH and controlled to a large extent by the chlorine to ammonia nitrogen (Cl: N) ratio. Temperature and contact time also play a role. Monochloramine is predominately formed when Cl: N is less than 5:1 by weight at Cl:N ratios above 7.6:1, free chlorine and nitrogen trichloride are present. Ozone (O3) is a powerful oxidizing agent that is very effective in killing bacteria and, in the process, is converted to oxygen, which improves water quality . When ozone is used, it is manufactured on site by passing oxygen or air through an electric discharge. Ozone disinfection, which is relatively expensive, is used quite widely in Europe but less frequently in the United States. Unfortunately, ozone can also generate by-products that may be associated with health risks. UV radiation is a relatively new disinfection technique and is effective in killing microorganisms: it is becoming increasingly popular in the United States as an alternative to chlorination. The UV source is a low-pressure mercury arc lamp. At most sewage treatment plants in the united states, DBPs can be k