CE520-lect8
advertisement

CE 420/520 Environmental Engineering Chemistry
Lecture 8 - Redox Reactions
Dr. S.K. Ong
Oxidation-Reduction Reactions
One of the more important reactions an environmental engineer will face in his career
Examples:
- organic oxidation, methane fermentation, nitrification and denitrification are redox reactions
mediated by microorganisms in wastewater engineering
- solubilization of metals such as iron and manganese
- chlorine and ozone added to water and wastewater for disinfection and transformation of organic
compounds
- fate of pollutants in the environment depends on the redox environment
- analytical tests used by environmental engineers are depend on oxidation-reduction reactions,
example, COD test
As with acid-base reactions, solubility and complexation reactions, oxidation-reduction reactions can also
be viewed from a state of equilibrium
An understanding of oxidation-reduction equilibrium can help indicate whether a particular reaction is
possible under given environmental conditions
Oxidation-reduction reactions are best visualized using half reactions where the transfer of electrons is
included in the equations
For example:
Soluble Cd2+ may be reduced and precipitated out by metallic iron in a wastewater as follows:
Cd2+ + Fe == > Cd + Fe2+
(1)
This reaction can be written as two half-reactions:
Cd2+ + 2e- < == > Cd
Fe2+ + 2e- == > Fe
Eo = -0.76 V
Eo = - 0.44 V
(2)
(3)
Eo is defined as the standard electrode potential for the above two reducing reactions. Since it is not
possible to determine the potential without a reference, the reduction of hydrogen ion to hydrogen is used
by convention as the reference:
H+ + e- == > 1/2 H2
Eo = 0.0 V at 25o C and 1 atm
See Table 7-1 for a list of Standard Electrode Potentials at 25o C. Note that by convention, the standard
electrode potential value is written for the reduction half-reaction.
Eo for a reaction may be related to the change in Gibbs Free Energy by the following equation:
where
Go = - nFEo
Go
= change in free energy (kcal/mole)
n
= number of electrons
Eo
= standard electrode potential (V) for the half-reaction
F
= Faraday’s constant = 23.06 kcal/volt-equivalent (or 96,500
coulombs/equivalent)
For the Cd – Fe reaction, the reaction standard electrode potential is obtained by subtracting equation 1
from 2.
Cd2+ + 2e- < == > Cd
Eo = -0.40 V
x -1
Fe2+ + 2e- == > Fe
Eo = - 0.44 V
Cd2+ + Fe == > Cd + Fe2+
Eo rxn= 0.04 V
1
If Eo rxn is positive, the reaction will proceed spontaneously as written
If Eo rxn is negative, the reverse reaction will occur spontaneously
By observing the Eo values, one can tell whether a particular reaction is more oxidizing than the other. The
relative potential of each oxidizing can be compared based on the Eo value. Example, ozone is a stronger
oxidizing agent than chlorine.
_______________________________________________________________________________
Redox reaction
Oxidizing
Eo (V)
Relative
Agent
at 25˚ C
Oxidative Power
O3 + 2H+ + 2e- = H2O
O3
2.07
1.52
HOCl + H+ + 2e- = Cl- + H2O
HOCl
1.49
1.10
Cl2 + 2e- = 2Cl
Cl2
1.36
1.00
The redox potential of a reaction can be related to the equilibrium constant K as follows:
G = Go + RTln Q
We have (see earlier notes)
dividing by – nF, we have:
- G/nF = - Go/nF - (RT/nF)ln Q
Erxn
= Eorxn – (RT/nF) ln Q
called the Nernst Equation
At equilibrium, E = 0 and Q = K, therefore:
Eorxn = (RT/nF) ln K
Example:
Permanganate (MnO4-) is used to oxidize the H2S in a wastewater. Calculate the
equilibrium constant for this oxidation-reduction reaction. Reaction at standard state is given by the
following half reactions. See Table 7-1
MnO4- + 8H+ + 5eS + 2H+ + 2e-
< == > Mn2+ + 4H2O
< == > H2S (g)
Eo = +1.51
Eo = +0.17
(1)
(2)
Equation (1) x 2, Equation (2) x 5, subtract equation (2) from (1)
5H2S + 6H+ + 2MnO4- < == > 2Mn2+ + 5S + 8H2O
Note that to obtain the Eorxn, we do not need to multiply the Eo values for the half-reactions by the 2 or –5
since the value of Eo is independent of reaction stoichiometry, i.e., the number of electrons involved in the
reaction. The potential will be the same even when reaction (2) is written as
1/2 S + H+ + e-
< == > 1/2 H2S (g)
Therefore:
Eorxn
= (1.51) –(+0.17)
= 1.34
Positive E : shows that reaction is spontaneous as written
o
ln K = nFEo/RT = (10)(23,000)(1.34)/(1.98)(298) = 522
K = e522 = 10 227
(implies reaction is irreversible as written)
2
Electron Activity Concept
Just as in an acid-base reaction where the acidity and basicity of the water are described by the use of the
pH or –log[H+], the degree of oxidizing or reducing conditions can be described by the use of the electron
activity concept.
Water with high electron activity such as in an anaerobic digester is said to be reducing. On the other
hand, water with low electron activity such as in a highly chlorinated water is said to be oxidizing.
As for pH, p (-log [e-]) is used to provide an indication of the oxidizing-reducing conditions of a system.
If p is large, [e-] is low, electron activity is low, conditions are oxidizing
If p is small, [e-] is high, electron activity is high, conditions are reducing
M2+ + e- < == > M+
General Equation
Then
K
[M ]
[e ] n [ M 2 ]
log[K ] log[e] log
[M ]
[M 2 ]
log[e] log[K ] log
Define
[M ]
[M n1 ]
p = -log[e-]
p o log[K]
Then:
p p 0 log
[M 2 ]
p p 0
In general
where
Example:
[M ]
p o
[reduc tan ts]
1
log
n
[oxidants ]
1
log[K ]
n
2HOCl + 2H+ + 2e- < == > Cl2 (aq) + 2 H2O
[Cl 2 (aq)]
1
1
p log K log
2
2
[HOCl ] 2 [H ] 2
p - pH or Eh - pH Diagrams
The relationship between p and pH or Eh and pH can be expressed graphically in the form of a p-pH or
Eh- pH diagram.
- Such diagrams show the regions of stability and the boundary lines for various species in water
- In addition, the p-pH diagram reduces the apparent complexity of an environmental system and
provides the engineer a better intuitive feel for the system he is working with
Examples of p-pH diagram
3
Construction of pe-pH diagram (Pourbiax diagram) (predominance diagrams)
1.
2.
3.
4.
5.
List all species
Pair the species up, write the appropriate redox or acid-base reaction and compute equilibrium constants
Obtain p-pH equations for each combination
Construct boundaries of solids if more than one solid exists
Construct boundaries of solids-solution species. Specify a given concentration for the total dissolved
component.
6. Construct boundaries of solution-solution species. At the boundaries, specify equal concentrations of
each species.
Example: Carbon system with H2CO3*, HCO3-, CO32- and CH4
1. Species - H2CO3*, HCO3-, CO32- and CH4
2. Pairs
H2CO3* - HCO3HCO3- - CO32H2CO3* - CO32H2CO3* - CH4
HCO3- - CH4
CO32- - CH4
3.Equations:
(a)
(b)
(c)
(d)
4. For H2CO3*
CO2(g) + H2O , == > H2CO3*
H2CO3* < == > HCO3- + H+
HCO3- < == > CO32- + H+
CO2(g) + 8H+ + 8e- < == > CH4 (g) + 2H2O
- HCO3- pair, use equation (a)
K
or
log K = -1.47
log K = -6.35
log K = -10.33
log K = 22.96
[HCO 3 ][ H ]
[H 2 CO *3 ]
-6.35 = log[H+] + log [HCO3-]/[H2CO3*]
pH = 6.35 + log [HCO3-]/[H2CO3*]
when H2CO3* = HCO3- at the boundary, then pH = 6.35, draw line at pH = 6.35
Limits of p in water
Two reactions determine the limits of p in water. There are:
Oxidizing – the oxidation of water to oxygen
2H2O < == > O2 + 4H+ + 4e-
Reducing – the reduction of water to produce hydrogen
2H2O + 2e- < == > H2 + 2OH- (2)
(1)
For equation (1), assume that the gas pressure of oxygen above water is 1 atmosphere, then:
p p o
1
log{[ H ] 4 [ PO2 ]}
4
For equation (1)
p = 20.8 - pH
At a specified pH, p values more positive than the one given in the above equation cannot exist at
equilibrium in water in contact with atmosphere. In a natural water system, with an oxygen partial pressure
of 0.21 atm and at a pH = 7.0, the p value is equal to
4
p = 20.8 + (1/4) log {[0.21]4[0.21]} = 13.8
For reducing water condition (equation 2), assuming partial pressure of hydrogen is equal to 1 atm:
p p o
1
log{[ H ] 2 [ PH 2 ]}
2
p = po + log[H+]
But po for hydrogen is equal to zero. Therefore:
p = -pH
At neutral pH, the lower limit is –7.0.
5
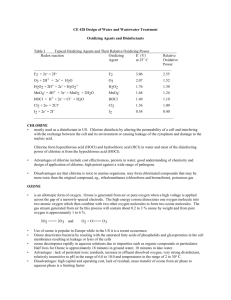
Oxidizing Agents and Disinfectants for Water Quality Control
Table 1 lists different oxidizing agents and their oxidation potential relative to chlorine. The oxidation
potential of an oxidant is related to its oxidation-reduction potential Eo (V). An oxidant with a high Eo is a
strong oxidant.
Table 1
Various Oxidizing Agents and Their Relative Oxidizing Power
_______________________________________________________________________________
Redox reaction
Oxidizing
Eo (V)
Oxidation
Chlorine
______________________________________________________________________________
F2 + 2e- = 2FF2
2.87
2.55
OH• + H+ + e- = H2O
OH•
2.33
2.05
Atomic oxygen
O
2.42
1.78
O3 + 2H+ + 2e- = H2O
O3
2.07
1.52
H2O2 + 2H+ + 2e- = H3O2+
H2 O 2
1.76
1.30
Perhydroxyl radical
HO2•
1.70
1.25
+
MnO4 + 4H + 3e = MnO2 + 2H2O
MnO4
1.68
1.24
+
HClO2 + 3H + 4e = Cl + 2H2O
HClO2
1.57
1.15
+
HOCl + H + 2e = Cl + H2O
HOCl
1.49
1.10
Cl2 + 2e = 2ClCl2
1.36
1.00
HBrO + H+ + 2e- =
HBrO
1.33
0.97
Br2 + 2e = 2Br
Br2
1.07
0.79
+
HIO + H + 2e = I + H2O
HIO
0.99
0.73
I2 + 2e- = 2II2
0.54
0.40
__________________________________________________________________________
CHLORINE
most prevalent disinfectant in US and used for oxidation and precipitation of Fe(II) and Mn (II)
for municipal wastewater (about 62% of total municipal wastewater is chlorinated (1982 US EPA Survey)
– use for odor control, disinfectant, ammonia removal, control of bulking in activated sludge system
in industry, example: control of biofouling in cooling towers and condensers, used as an oxidizing agent
for cyanide (CN-) .
Commonly available form
chlorine gas – yellowish-green gas
- vaporizes at –34.5o C,
- slightly soluble in water with a maximum solubility at 1atm of approx. 1% at 9.6 o C.
- concentrations of 15 - 20 ppm for 30 - 60 minutes can be dangerous, higher concentrations are fatal.
Salts of hypochlorous acid
dry – calcium hypochlorite (Ca(OCl)2)
liquid – sodium hypochlorite (NaOCl) – concentration between 5 to 15% available chlorine.
Reactions of chlorine
Chlorine form hypochlorous acid (HOCl) and hydrochloric acid (HCl) in water and most of the
disinfecting power of chlorine is from the hypochloride ion (OCl -).
Cl2 (aq) + H2O < == > HOCl + H+ + Cl-
6
Note change in oxidation number for chlorine (referred to as disportionation or autooxidation) and the
change in pH (alkalinity is consumed).
Addition of sodium hypochlorite to water results in
NaOCl + H2O < == > HOCl + Na+ + OHNote that pH will increase slightly
The distribution between HOCl and OCl- is given by the following:
HOCl < == > H+ + OCl-
pKa = 7.5
For disinfection, HOCl is about 50 to 100 times more effective as a disinfectant than OCl- (see diagram).
Chlorination of Water
Chlorine Demand - chlorine consumed when added to water before a residual of chlorine appears
Chlorine demand = chlorine added - chlorine residual
(i) sunlight – aqueous chlorine solution is not stable when exposed to sunlight, UV provides
energy for the conversion of hypochlorite to chloride (example in a swimming pool)
UV
2HOCl < === > 2H+ + 2Cl- + O2
(ii) reactions with organics
such as humic material, organic compounds which are converted to chlorinated
compounds or carbon dioxide depending on the pH. Of importance is the formation of
trihalomethanes (THM) or halogena substitutes of the simplest organic molecule,
methane, example. Chloroform CHCl3, dichlorobromoform – CHBCl2.
(iii) Reactions with inorganics
Cl2 (aq) + 2 Fe2+ < == > 2 Fe3+ + 2 ClHOCl + H+ + 2 Fe2+ < == > 2Fe3+ + Cl- + H2O
(reactions are almost instantaneous at neutral pH)
NO2- + HOCl < == > NO3- + Cl- + H+
Zero
Chlorine demand
Chlorine residual
(mg/L)
chlorine dose (mg/L)
chlorine demand
7
Reactions with Ammonia and Amines
ammonia in wastewater is usually in the range of 10 to 40 mg/L, sources of ammonia is from urea
present in urine.
(NH2)2C=O + H2O = > 2NH4+ + HCO3- + OHNH3 + HOCl == > NH2Cl + H2O
NH2Cl + HOCl == > + H2O
NHCl2 + HOCl == > NHCl3 + H2O
2NH3 + 3HOCl == > N2 (g) + 3HCl + 3H2O
monochloramine
dichloroamine
trichloramine (nitrogen trichloride)
All the above reactions are occurring simultaneously - how far these stepwise reactions proceed and
how much of each compound is formed depends on the pH, temperature and time of contact and
reactant concentrations.
Low pH, high Cl2:NH3 ratio - favor dichloramine
pH > 8.5, favors monochloramine
pH between 5.5 to 8.5 - monochloramine and dichloramine
pH between 4.5 and 5.5 - dichloramine
Below pH 4.4 - nitrogen trichloride will be produced
Note high Cl2: NH3 ratio 15:1 on a molar basis - gaseous nitrogen is the principal product.
The term Combined available chlorine is used for chlorine existing in chemical combination with
ammonia or organic nitrogen (chloramines, NH2Cl – monochloramine, NHCl2 – dichloramine, NHCl3 trichloramine).
In the last reaction above, when N2 is formed, the amount of combined available chlorine will
decrease. For higher chlorine dosage, free available chlorine will appear.
Free Available chlorine – chlorine existing in the form of hypochlorous acid and/or hypochlorite ion
The point where free chlorine appears is called the breakpoint. Note that a distinct breakpoint is
usually not observed with ammonia and organic nitrogen present. Reason is that organic nitrogen
continues to be oxidized and hydrolyzed - providing ammonia and therefore chloramine will be present
even after the so-called breakpoint. Most water treatment systems will add chlorine until free available
chlorine are present. Called breakpoint chlorination.
Look at the generalized diagram.
Industrial Application
• A common application of chlorine in waste treatment is the destruction of cyanide wastes. This
process is called alkaline destruction and is commonly used in metal plating industry. The reaction is
sensitive to pH with a pH greater than 10.
Step 1 - cyanide is oxidized to a less toxic cyanate
NaCN + Cl2 + 2NaOH ==> NaCNO + 2NaCl + H2O
Step 2 - cyanate is further oxidized to carbon dioxide and nitrogen
2NaCNO + 3Cl2 + 4 NaOH ===> 2CO2 + N2 + 6NaCl + 2H2O
•
Advantages of chlorine include cost effectiveness, good understanding of chemistry and design of
application of chlorine, high potent against a wide range of pathogens.
8
•
Disadvantages are that chlorine is toxic to marine organisms, may form chlorinated compounds that
may be more toxic than the original compound, eg., chlorination of phenol forms chlorinated phenolic
compounds that are more toxic than phenol.
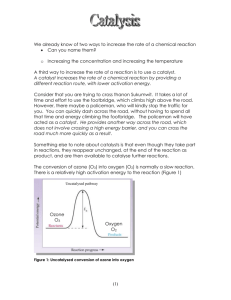
OZONE
• is an allotropic form of oxygen. Ozone is generated from air or pure oxygen when a high voltage is
applied across the gap of a narrowly-spaced electrode. The high energy corona dissociates one oxygen
molecule into two atomic oxygen which combine with two other oxygen molecules to form two ozone
molecules. The gas stream generated from air by this process will contain about 0.2 to 3 percent ozone by
weight and from pure oxygen is approximately 1 to 6 percent.
3O2 <==> 2O3 and
O2 + O<==> O3
127±3o
0.126 nm
- 0.224 nm
• ozone is generated as a gas, therefore treatment of aqueous contaminants require the transfer from the gas
to liquid phase (KH = 0.082 atm•m3/mole)
• ozone decomposes rapidly in aqueous solutions due to impurities such as organic compounds or
particulates. Half lives for ozone is approximately 18 minutes in ground water and 10 minutes in lake
water.
• oxidation of compounds in aqueous solution comes from
• direct oxidation reaction with molecular ozone
• indirect pathways in which free radicals, primarily hydroxyl (OH•) radicals or hydroperoxide
(HO2•) radical
Note that most ozone reactions involved a chain reaction involving OH• radicals. The reaction rate
constant for the destruction of organics by OH• is typically several orders of magnitude greater than for
O3 alone.
• molecular ozone oxidation is selective with second order rate constants (with respect to ozone and
reduced species constant) between 1 - 103 M-1s-1
- kinetics of OH• reaction are several orders of magnitude faster than molecular ozone oxidation
• The reactions of ozone in pure water is not completely understood. The initial step results in the hydroxyl
radicals making alkaline oxidation by ozone several orders of magnitude higher than ozone oxidation in
acidic solution.
(O3- is ozonide ion, O2- superoxide ion)
• In a typical aqueous solution, there are:
initiators - promotes decomposition of ozone to form radicals, eg., H 2O2, OH-, Fe2+, UV, formate
and humic acid
promoters - reacts with OH• to form radical species, resulting in propogation of reactions,
scavengers - react with hydroxyl radicals to form secondary radicals which do not promote
reaction but rather quench the chain reaction.
9
• Advantages with ozone
¶ lack of persistent toxic residual
¶ increase in effluent dissolved oxygen concentrations
¶ as a disinfectant it is relatively insensitive to pH in the range of 6 to 10 and temperature in the
range of 2˚ C to 30˚ C.
• Disadvantages are
¶ high capital cost and generation cost (approx. $2,400/kg/day for a plant producing 900 kg/day)
¶ mass transfer of ozone from air phase to aqueous phase is a limiting factor
¶ use of UV to enhance oxidation may be affected by the presence of particles and turbidity of the
wastewater
HYDROGEN PEROXIDE
• Hydrogen peroxide by itself is slightly more powerful than chlorine. As in ozone, the oxidizing agent is
the hydroxyl (OH•) ion. Hydroyl ions from hydrogen peroxide may be formed from several methods.
H2O2 + hv ===> 2OH•
¶
Irradiation of UV
¶
Metallic Catalysts - Ferrous ions (Fenton's reagent) or a fixed catalyst eg., titanium oxide
or
Fe2+ + H2O2 ==> Fe3+ + OH• + OHFe2+ + OH• ===> Fe3+ + OH2Fe2+ + H2O2 + 2H+ ==> Fe3+ + 2H2O
The rate law is
-d[H2O2]/dt = k [H2O2][Fe2+]
This suggests that the mechanism may be :
H2O2 + Fe2+ ===> Fe(OH)2+ + HO•
HO• + Fe2+ ==> Fe()H)2+
Fe(OH)2+ + H+ ===> Fe3+ + H2O
with the first reaction the rate limiting reaction.
The above equation implies that Fenton's reagent is strongly dependent on solution pH. In fact, in
acidic conditions, OH• is the predominant reactive oxidant. The decomposition of hydrogen peroxide
reaches a maiximum at a pH of 3.5.
¶
ultrasound - energy is imparted to hydrogen peroxide in the similar manner as uv to form
hydroxyl radicals.
10