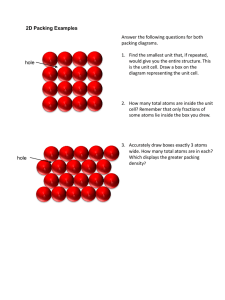
Solids
advertisement
GENERAL CHEMISTRY 115 SIMPLE CRYSTAL STRUCTURES I. Unit Cells: The unit cell is defined as the smallest unit of a repeating pattern that contains all of the geometric information. This means that replicating the structure in all directions, by placing identical unit cells next to each other, will recreate the full structure. For example, Fig. 1 below shows two systems that repeat and identifies the unit cell for each. The unit cells in each pattern are identified using heavy black lines. That from the 2nd drawing is also shown at the right of the repeating pattern. Dotted or dashed lines show how those unit cells repeat to create the full pattern. In the first drawing, the structure repeats in only one direction – from left to right – and in the 2nd drawing, it repeats in two directions – left-right and up-down. Three dimensional crystal structures will, of course, repeat in three directions, as we will see in the next section. Fig. 1: Two repeating patterns and their unit cells. A B C A B C A B C A B C II. The Cubic Structures: In Fig. 2, diagrams are shown that represent three of the cubic unit cells. These drawings use small atoms at each site so that their positions are clear. To aid in viewing, the outline on the front face is done with a heavier line and the atoms positioned at the back are shown slightly smaller in size, compared to those in front, in order to mimic perspective. Note that in the 3rd structure, 3 atoms are not shown. The ones shown are all the corner atoms, and 3 of the 6 face atoms, (those positioned in the middle of each face). Shown in the drawing are the front, top, and right face atoms, but not the bottom, left, or back face atoms. In metals that form crystals using these unit cells, the closest distance between any two occupied positions will be the direction that establishes where the atoms are “in contact” – i.e., where their radii touch, (if atoms were actually hard spheres). –1– Raynor: 2/6/2016 Fig. 2: The Cubic Unit Cells Simple Cubic Body-Centered Cubic Face-Centered Cubic All three crystal structures have atoms occupying each of the 8 corner positions. In the 1st structure, these are the only occupied positions. This structure is called the simple cubic unit cell, (or the primitive cubic unit cell). The 2nd structure has atoms at the 8 corners, but also has a single atom located at the center of the cube, (inside the “body” of the unit cell), and is therefore referred to as the body-centered cubic unit cell. [The body atom is shown using a lighter shade of gray.] Finally, the 3rd structure has atoms placed in the middle of each face on the cube, as well as the atoms at the 8 corner positions, and is therefore called the face-centered cubic unit cell. There are a total of 6 faces on a cube – one at top, at the bottom, at the left, at the right, at front, and at back. Thus this structure will have one atom positioned in the middle of each of these 6 faces. [As previously noted, only 3 of these face-atoms are shown in the drawing above; these atoms are shown using a lighter shade of gray.] These structures represent 3-dimensional unit cells. They are the smallest repeat units occurring in the full 3-D crystal. To create the full macroscopic crystal, we simply line up unit cells so they are touching each other, replicating them in every direction – i.e., left, right, up, down, in, and out. III. Closest-Packed Structures: For completely spherical systems, (such as in atomic crystals), the best way to pack identical spheres is the same way we would stack oranges or grapefruits in a display in the grocery store. Let’s consider how we might stack identically-sized spheres. We could stack them using the method shown in Fig. 3, below, with each sphere touching only 4 others in each layer – i.e., each sphere is in contact with one on its left, one on its right, one above and one below. Fig. 3: Simple cubic packing of spheres. If we then lay another identical layer on top of this one, we get the identical structure as in the simple cubic arrangement illustrated in Fig. 1. Note that the center sphere, (shown in a lighter shade in Fig. 3), will have exactly 6 nearest neighbors – i.e., it is in contact with 6 other spheres, (one to its left, one to its right, one up, one down, one in, and one out). However, this arrangement wastes a lot of space, as can be seen in Fig. 3. A much more efficient arrangement is to use what is called a “closest-packed” arrangement of the spheres. We start with –2– Raynor: 2/6/2016 a layer of spheres arranged so that each central sphere is surrounded by 6 other spheres, as shown in Fig. 4, (where the central sphere is shown in a lighter shade of gray). [Mathematically, it can be shown that exactly 6 spheres can be in contact with the central sphere and in contact with each other.] Note how much less empty space occurs between the spheres in Fig. 4 than between those in Fig. 3. Let’s define the arrangement in this layer as Layer B: Fig. 4: Closest-packing of spheres around a central sphere. 1 2 6 3 5 4 Layer B If we wish to minimize the empty space around each sphere, we can stack the spheres in the next layer, (either in a layer above or below the first one), so that the spheres are nestled within the holes formed in the first layer. There are 6 such holes around the central atom, which have been numbered in Fig. 4 from 1 to 6. [The holes are those seen immediately around the center atom.] However, we cannot place 6 spheres in those holes – they would not fit. We can fit exactly 3 spheres in the holes above the center atom, such that each new sphere touches the central atom and fits in one of the holes formed around it. This leaves 3 holes unused, because of the size of the spheres. As shown in Fig. 5, there are two different ways we can position the next layer after that. We can position it so that spheres occupy holes 1, 3 and 5, leaving holes 2, 4 and 6 unoccupied, or we can position it so that spheres occupy holes 2, 4 and 6, leaving holes 1, 3 and 5 unoccupied. We will call these two possible choices as Layer A and Layer C as indicated below: Fig. 5: Possible positions for spheres in layer above Layer B. Layer A Layer C This creates two different repeat patterns that can be made when we stack layers to create the 3dimensional closest-packed structure. The first way is to simply alternate between layers A and B, (or to alternate B and C), to create an ABABAB… arrangement. This is called a hexagonal closest-packed structure. Note that this leaves 3 holes that are never used – i.e., if we look down our final structure, we will see periodic holes where no spheres are seen, no matter how many layers have been added. Alternatively, we can alternate layers using all three forms so that the order creates the following repeat pattern: ABCABCABC… This is called a cubic closest-packed structure. Using this pattern, each hole is used as we stack layers. If we were to look through all the layers, we would find that no holes persist through the entire structure. –3– Raynor: 2/6/2016 Although it is not easy to see, the cubic-closest packed structure is actually identical to the facecentered cubic structure. [The book shows a nice picture that helps show that this is true.] However, the hexagonal closest-packed structure is not identical to any of the 3 cubic structures shown originally – it provides a 4th type of arrangement, (one that is not based on any cubic type of unit cell). We can now easily determine the number of nearest neighbors for each sphere in a closest-packed arrangement. As shown in Fig. 3, each sphere is in contact with 6 others within its original layer. Then it is in contact with 3 others in the layer stacked above its layer and with 3 more in the layer stacked below its layer. Thus, regardless of which type of closest-packed arrangement it is in, each sphere will be in contact with 12 others. IV. The Coordination Number: The coordination number of a crystal is the number of nearest neighbors that any atom, (or molecule or ion), has in the structure. As shown above, atoms (or molecules) in closest-packed structures have 12 nearest neighbors. Thus the coordination number for their crystal type will be 12. We can therefore conclude that the coordination number for face-centered cubic structures will be 12, since this type of unit cell is identical to cubic-closest packing. To determine the coordination number for the other 2 unit cells, we first need to determine which spheres will be in contact with one another within the unit cell. For the simple cubic structure, each sphere is in contact with the ones next to it along the edges of the cell. I.e., each atom is touching one to its right, one to its left, one above, one below, one in front and one in back. Thus the coordination number will be 6 in simple cubic structures. In the face-centered cubic structure, the atoms are in closest contact along the face diagonals – i.e., along the diagonal line connecting two atoms on opposite sides of a face, as shown in Fig. 6. Fig. 6 shows the 4 corner atoms that are in contact with a face-centered atom, (which is shown in a lighter shade of gray). Dashed lines indicate the lines along the face diagonals. Fig. 6: Contact along a face diagonal. Note that although the atoms touch one another along the face diagonals, they are not in contact with the atoms next to them along the edges. Although it is easy to see that the atom in the middle of the face is in contact with 4 of the corner atoms, it is not easy to see which other atoms in the 3-dimensional structure will be in contact with it. However, as indicated earlier, the face-centered cubic structure is identical to cubic closest packing. As we saw, this means that there will actually be a total of 12 atoms in contact with the center atom. Thus, the coordination number is 12 for face-centered cubic structures. –4– Raynor: 2/6/2016 Finally, for the body-centered structure, the closest contacts occur between atoms on the body diagonal – i.e., the line from the bottom-left-front atom to the top-right-back atom or the bottomright-front atom to the top-left-back atom, etc. All of these body diagonals pass through the central body atom. Thus the atom drawn in the center of the unit cell will ultimately be touching all 4 of the atoms at the corners above it and all 4 of the atoms at the corners below it, giving a coordination number of 8 for this structure. IV. The Number of Atoms inside a Unit Cell: If we stack together cubic unit cells in every direction, we will see that each corner atom is shared by 8 different unit cells. For example, if we consider the atom at the bottom left corner of the picture shown for simple cubic structures, that atom will be shared with the unit cell stacked to its left, the one stacked below it, and the one stacked below and to its left, as shown in the drawing below: Note that it is shared by 4 different cubic unit cells that are in this one layer. Now, if we stack a layer of unit cells on top of this one, the atom will become shared with 4 more unit cells, for a total of 8 unit cells. Thus 1/8 of this atom will belong to each of those 8 different unit cells. Each corner in the cubic unit cells contains an atom. Since there are 8 such corners and each corner atom is 1/8 within that unit cell, the total contribution from corner atoms will be 8 1/8 = 1 atom per unit cell. Any atom that is inside the body of a unit cell, (such as the center atom in the body-centered unit cell), belongs only to its own unit cell. Thus the body atom in a body-centered cubic structure contributes 1 atom to its unit cell. When we stack unit cells together, any atom that is in the middle of a face will be shared by 2 different unit cells. Thus ½ of it will belong to each of those unit cells. Since each unit cell has a total of 6 different faces, (one at the top, one at the bottom, one at the left, one at the right, one in front, and one in back), there will be a total of ½ 6 = 3 atoms within any cubic unit cell having atoms at face positions. Finally, if a unit cell has atoms along the edges of its cell, those atoms are shared by 4 different unit cells, (after stacking unit cells together). Since a cube has a total of 12 edges, the contribution from edge atoms will be ¼ 12 = 3 atoms per unit cell. [In the three structures we studied, none of them possess atoms along the edges. However, some more complicated structures will have edge positions that are occupied.] –5– Raynor: 2/6/2016 The table below summarizes these results: Table 1: Number of Atoms Per Unit Cell by Position in Unit Cell Position of Atom Corner Face Body Edge Fraction of Atom Per Unit Cell 1 8 1 2 1 1 1 4 # Positions Per Unit Cell Net # Atoms Per Unit Cell 8 1 6 3 1 1 12 3 Using this information, we can determine the total number of atoms per unit cell in each of the three cubic structures presented at the beginning. First, all three structures have atoms on each corner and thus get 1 atom per unit cell from the corner atoms. Since the simple cubic structure has no other types of atoms, it will contain 1 atom per unit cell. The body-centered cubic structure has an atom inside the body of the unit cell, in addition to the corner atoms. Thus a body-centered cubic structure will have total of 2 atoms per unit cell. Finally, a face-centered cubic structure has atoms on its faces, (3 atoms per unit cell), as well as at the corners, (1 atom per unit cell). Thus a face-centered cubic structure will have 4 atoms per unit cell. V. Determining Atomic Radii from Edge Lengths in Unit Cells: Using X-Ray Crystallography, we can determine not only the type of unit cell formed by the crystal, but also the lengths along each edge of the unit cell and the angles formed by those edges. [In cubic unit cells, the angles are all 90 and the edges all have the same length.] Once we know this information, we can use it to determine the value for the atomic radii of any element that crystallizes as single atoms, such as metals. To determine atomic radii, we first need to establish the direction along which the atoms will be in contact, (i.e., the direction between nearest neighbors). First, recall that the atoms in simple cubic structures are in contact along the edges; atoms in body-centered cubic structures are in contact along the body diagonal; and atoms in face-centered cubic structures are in contact along face diagonals. Using this information, and knowing the length of an edge in the unit cell, a, we can determine the atom’s atomic radius. Simple Cubic: Shown are 2 atoms that are touching each other along an edge of the unit cell. Note that the edge length, a, contains 1 radius from the left atom and 1 radius from the right atom. Thus, 2r = a. This gives the following relationship for the radius of the atom: r = ½ a. r r Face-Centered Cubic: The atoms in face-centered cubic structures touch along the face diagonals – i.e., from one corner, through the center face atom, to the opposite corner. One of these face diagonals is shown in the drawing –6– Raynor: 2/6/2016 at right, using a dashed line. The length of this line, d, can be shown to be d 2a , where a is the length of each edge in the unit cell. Fig. 6 shows how the atoms are arranged when they touch along a face diagonal. We can see that 3 atoms lie on this face diagonal. The one at the bottom-left-front corner and the one at the top-right-front corner each have only one of their radii on the line, (the other lies inside another unit cell), but the one in the middle of the face has two of its radii on the line. Thus there are 4 radii along this line and we can equate 4 radii with the length of the face diagonal. I.e., d 4r 2a . Solving for the radius of an atom gives r 2 / 4 a 1/ 8 a Body-Centered Cubic: A body diagonal is any line that connects from one corner, through the center of the unit cell, to an opposite corner. In the drawing, one such body-diagonal is shown. It connects from the bottom- right-front corner to the top- left-back corner. The length of this line, d, can be shown to be given by d 3a , where a is the length of each edge in the unit cell. If we look at how the atoms are packed in this structure, we find they have contact along this body diagonal. Just as with the face-centered cubic structure, there will be 3 atoms that touch each other along that body diagonal: the one at the bottom-right-front corner; the one in the middle of the body; and the one at the top-left-back corner. They are shown above. Just as with the face-centered cubic structures, there will be a total of 4 radii along this line. [One from the atom on the left, one from the atom on the right and two from the atom in the middle.] This gives d 4r 3a . Solving for the radius of the atom, we get the following: r 3/4 a. VI. Determining the Density of a Solid from its Cell Dimensions: Recall that the density of a pure substance is defined to be the ratio of its mass to its volume. In pure substances, this density remains unchanged, regardless of the size of the sample we might take. Thus we can determine the density of a uniform solid from the density of its unit cell: dsolid mass of unit cell . volume of unit cell All three of the structures we are considering are cubes. Since the volume of a rectangular solid is given by the product of its length times its height times its depth, the volume of a cube, where the length = height = depth = a, will be a3. I.e., V a 3 , where a is the length of one of the edges in the unit cell. The mass of the unit cell depends upon the number of atoms inside the unit cell and the mass of each atom. I.e., mass of unit cell = (# atoms per unit cell) (mass of 1 atom). We saw earlier that simple cubic structures have 1 atom per unit cell, body-centered have 2 atoms per unit cell and face-centered have 4 atoms per unit cell. The mass of a single atom can be determined directly from the atomic weight of the atom, since the atomic weight is the weight in grams of 1 mole of the substance – i.e., the weight in grams of 6.022 1023 atoms. Thus the weight of a single atom will be given by AW/6.022 1023. Putting these ideas together, we get the following formula for the density of a solid: –7– Raynor: 2/6/2016 dsolid # atoms per unit cell AW/6.022 1023 a3 . If we know which kind of unit cell is involved and we know the length of an edge in that unit cell, we can then determine the density of that substance in the solid phase. [Or, given the kind of unit cell and the density of the metal, we can determine the length of an edge.] Caution! Make sure you convert the units of a to cm before you cube it! You need your units of density to be g/cm3! [Note also – do not forget to cube all parts of the value of a, including its units. For example, consider the value of a3, when a = 2.00 10–8 cm: a 3 2.00 108 cm 2.00 10 cm 3 3 8 3 3 8.00 1024 cm3 ] VII. Summary of Results: Table 2 summarizes the results discussed earlier for crystalline systems. Table 2: Properties of Cubic Unit Cell Structures (a = length along an edge) Unit Cell Direction of Closest Contact # Atoms/Unit Cell Coord. No. Simple cubic Edge 1 6 Body-Centered Body Diagonal 2 8 Face-Centered Face Diagonal 4 12 –8– Length of Radius, r 1 r a 2 3 r a 4 1 r a 8 Raynor: 2/6/2016