Solid Crystal Structures
advertisement

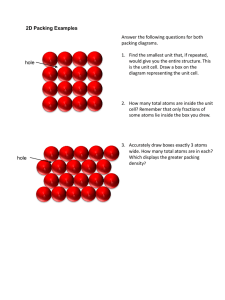
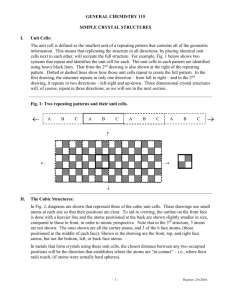
Solid Crystal Structures (based on Chap. 12 Sec 11 of Jespersen 6th Ed) Dr. C. Yau Fall 2014 1 What are Crystals? They are solids made of particles (atoms, ions or molecules) that are arranged in a rigid, orderly 3-dimensional array often referred to as a lattice: the crystal lattice. Link to some neat videos of formation of crystals: http://www.youtube.com/watch?v=uoexANHeWoU&feature=related http://www.youtube.com/watch?v=HnSg2cl09PI&feature=related http://www.youtube.com/watch?v=xTIzMaSDZ3k 2 The unit cell is the smallest repeating unit in the lattice. Simple Cubic Cell: Atoms are drawn smaller to show 3-D layout. Actual size is such that corner atoms touch. 3 Simple Cubic Cell Polonium (Po) has the simple cubic cell structure. Each unit cell contains 8 corners, with one atom at each corner. Each atom is shared by how many cells? Thus, only 1/8 of the atom is in a cell. 4 What is the # of atom per cell? Figure shows how an atom at the corner of a cubic cell is shared by 8 cells. Therefore only 1/8 of the atom belongs to a cell. 5 Face-Centered Cubic Cell Cu, Au, Ag and Al have the face-centered cubic cell structure. Each unit cell has an atom at each corner and one at the center of each face. How many cells share the atom at the center of the face? 6 What is the # of atoms per cell? Face-Centered Cubic Cell The figure shows how an atom at the center of a face is shared by 2 cells. It therefore counts only as ½ of an atom. Note that the corner atoms don’t touch, but each corner atom touches the atom at the center of the face. 7 Body-Centered Cubic Cell Cr, Fe, Pt have the body-centered cubic cell, with one atom at each corner plus one in the center of the cube. What is the # of atoms per cell? Note that corner atoms don’t touch but each corner touches the atom at center of the cell. 8 • So far we examined only metals, where all the lattice points are of the same species. • Copper has only Cu atoms. • Silver has only Ag atoms. • How are ionic cmpds different? Lattice points are occupied by two different species: the cation & the anion. 9 Rock Salt Structure Fig.12.40: NaCl crystal. Chloride ions are in a facecentered cubic cell with sodium ions between them, plus one in the center of the each cube. (Center Na+ not visible here) 10 What is the number of Na+ and Cl- per cell? Unit Cell of Cesium Chloride Fig. 12.41: The unit cell for cesium chloride, CsCl. Cl- is located in the center of the unit cell. Cs+ are at the corners of the unit cell. Note: Ions are not drawn to full size. What is the formula based on the crystal structure? 11 Zinc Blende Structure S2- has the face-centered cubic cell structure. There are 4 Zn2+ inside the cell. What is the formula based on the crystal 12 structure? Calcium Fluoride Crystal Structure Ca2+ is in a face-centered cubic cell structure. There are 8 F- inside the cell. What is the ratio of Ca2+ to F- based on the 13 crystal structure? Determining Formula from Crystal Structure A new compound, boganium sulfide, has been discovered. X-ray crystallographic studies reveal that it has a cubic unit cell with a sulfide ion forming a body-centered unit cell and a boganium ion (Bo) in the center of each of the cube faces in the unit cell. Based on this structure, what should the formula for the compound be? 14 Amorphous Solids Not all solids are crystalline. Some are “amorphous.” Glass is noncrystalline. 15 X-Ray Crystallography Fig 12.49: X-ray diffraction: X-ray hitting a crystal is diffracted & gives a characteristic diffraction pattern (b). Shown is the diffraction pattern of NaCl on a photographic film.. You do not need to know Bragg equation. Just know that X-Ray crystallography tells us the type of crystal 16 structure and the distance between the atoms. Example 12.7 p.570 X-Ray diffraction measurements reveal that copper crystallizes with a face-centered cubes lattice in which the unit cell length is 3.62Ǻ. What is the radius of a Cu atom expressed in angstroms and in picometers? (1nm = 10 Å, 1m = 1010 Å; 1m = 1012 pm) m _ _ mm _ _ m _ _ nm Å _ _ pm Remember that the corner atoms do not touch each other in the face-centered cell. 17 Paper HW (due date will be announced). Barium has a body-centered cubic structure. If the atomic radius of barium is 222 pm, what is the density (in g/cm3) of solid barium? Hint: D of the unit cell = D of the solid. Remember D = M/V. Find the volume of the unit cell. Determine # atoms per cell and calculate the mass in g of those atoms (not moles of atoms). Remember to review which atoms touch which in a body-centered cubic structure. 18 Hint for Paper HW(continued) c a b a To determine the volume of a unit cell, you need to know the length of the cell (a). a First calculate c from the atomic radius. Write a Pythagorean theorem equation for the triangle with c as the hypotenuse in terms of a and b. Next, write the Pythagorean eqn for the triangle with b as the hypotenuse in terms of a. Finally, solve for a. 19 Physical Properties of Solids • Ionic crystals have cations and anions at the lattice points. • Molecular crystals have neutral molecules at the lattice points. • Covalent crystals have atoms at the lattice points, covalently bonded to neighboring lattice points. These are often called “network solids.” • Metallic crystals have cations at the lattice 20 points. Network Solids quartz diamond = oxygen = silicon 21 Electron Sea Model of Metal This model describes the metal as cations surrounded by a sea of valence electrons. The sea of electrons acts as a buffer between the positive ions, thus allowing it to be malleable, ductile and able to conduct electricity. 22 Table 12.7: Types of Crystals Study the table on p.573. It compares the type of attractive forces and typical properties of various types of crystals: • ionic • molecular • covalent (network) • metallic 23 Example 12.8 p.574 The metal osmium, Os, forms an oxide with the formula OsO4. The soft crystals of OsO4 melt at 40 C, and the resulting liquid does not conduct electricity. To which crystal type does solid OsO4 probably belong? Do Practice Exercise 19, 20, & 21 p.574 24