ionic bond
advertisement

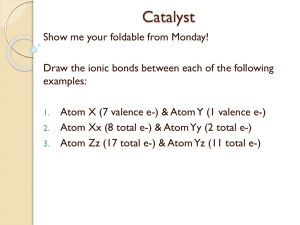
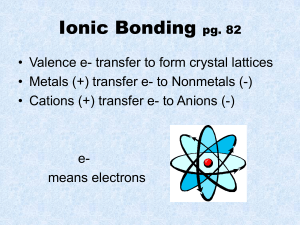
Directions Write “No Catalyst” on Catalyst sheet Take out a pencil and a red pen Create test barrier with arts binder Put everything else away Periodic Table Test • • • • • Use pencil Follow directions When done, bring up to me Complete notebook check Tape on inside front cover, put notebook on shelf • Get article from me, start homework Take Out Get science notebook from shelf Catalyst sheet Pencil Homework Catalyst North Side of Table Pretend that you’re the size of an atom, observing a reaction between a potassium atom, and a fluorine atom. Write an account of the formation of an ionic bond as the atoms react. Tell what happens to the valence electrons on each atom and how each atom is changed by losing or gaining electrons. South Side of Table Pretend that you’re the size of an atom, observing a reaction between a fluorine atom, and another fluorine atom. Write an account of the formation of an covalent bond as the atoms react. Explain what happens as the two atoms come together. When you’re finished, tape the papers I give you onto p. 54 & 55 Bonding Activity • Work as a team to follow directions on page 54 • Everyone must complete pages 54 and 55 • Clean up any mess made around the sink • Be careful with glassware • When everyone in the group has completed pages 54 and 55, raise your hands Exit Ticket Of the substances that you observed today (Distilled water, Table Salt, Sugar, Vegetable Oil, Isopropyl Alcohol), which do you think are held together by ionic bonds, and which do you think are held together by covalent bonds? Catalyst 1. Explain how metal atoms form metallic bonds in crystals. 2. What role do the valence electrons play? 3. How does a metallic bond differ from an ionic bond?