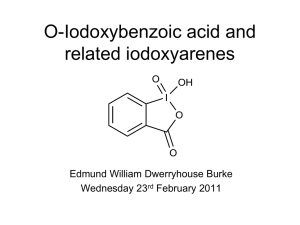
Diketopyrrolopyrroles DPP
advertisement

Diketopyrrolopyrroles DPP Cl H N O O Marek Grzybowski Cl N H Introduction • Brilliant red or orange high performance pigments • Extremely: H Insoluble Resistant (ΔT, hν, etc.) • Used in: O N N H O Luxury cars painting High quality printing Plastics coloring Solar cells, OLED, Fluorescence imaging etc. History 1974 – Farnum et al – accidental discovery of DPP1 H N O CN Zn + O O H N Br OEt N H 1. Farnum D G, Mehta G, Moore G G I, Siegal F P, Tetrahedron Lett. 1974, 15, 2549 O 5-20 % N-Unsubstituted DPP - History 1983 – Iqbal and Cassar patent2: Synthesis of DPP by condensation of succinate with aromatic nitrile CN H N O OR + OR 1 R2ONa, R2OH 1 T O R1 , R2 = iPr, tBu, tAm etc. 2. Iqbal A, Cassar L, Ciba-Geigy Ltd, US Patent 4, 1983, 415, 685 O O N H 30-70 % N-Unsubstituted DPP - Insolubility Reason: Strong molecular interactions3,4 7° 1,82Å 3. Lenz R, Wallquist O, Surface Coatings International Part B: Coatings Transactions, 2002, 85, 1-xxx 4. Mizuguchi J, Grubenmann A, Wooden G, Rihs G, Acta Crystallographica, Section B, 1992, 48, 696 N-Unsubstituted DPP - Insolubility Reason: Strong molecular interactions3,4 3,5Å 3,5Å 3. Lenz R, Wallquist O, Surface Coatings International Part B: Coatings Transactions, 2002, 85, 1-xxx 4. Mizuguchi J, Grubenmann A, Wooden G, Rihs G, Acta Crystallographica, Section B, 1992, 48, 696 N-Unsubstituted DPP – Properties Heat fastness: < 500°C 8th grade light resistance (1 to 8 scale) ε > 30000 M-1 · cm-1 Low Stokes shift < 30 nm (< 0,75 eV) usually ~12-15 nm λmax: 500-550 nm Hypsochromic shift in solution Fluorescence quantum yield ~0,5 110 mg dissolves in 1L of DMF Commercially available DPP pigments5 ~ 2000 tonnes / year -Ar = Ferrari RedPigment (Pigment Red 254) Red 255 Pigment Red 272 H N O Cl H N CH3 „Ciba was selling O Ar this for $100 per kilo, and the cost wasAr$20 per kilo, soPigment it was a big advantage for the Red 254 Pigment Red 264 6 N company„ O H Cl N H O Cl Pigment Orange 73 Pigment Orange 71 CN 5. Industrial Organic Pigments, Herbst W, Hunger K, WILEY-VCH, Weinheim, 2004, p.490 6. http://blog.cleveland.com/pdextra/2007/10/pollock_cuts.html Modifications R O N S O N ‚Latent pigment’ RO H N S N H R alkylation O Lawesson reagent N O acylation POCl3 OR O H N ArNH2 O N O O H N Heating N H O N H sulfonation bromination O Br Br H N N H O HO 3S 6. Zambounis J, Hao Z, Iqbal A, Nature, 1997, 388, 131 O SO 3H H N N H O N Ar N-Alkylation Insoluble becomes soluble Ar HN O NH Ar RX, DMF K2CO3 R N O N R 120 oC O Ar 5-90 %7 O Alcoxides and hydroxides were also used as bases Ar Larger/More branched substituent = Better solubility ΦF increases up to 0,9 Stokes shift up to 70 nm (~2 eV) Lower temperature resistance (<300°C) 7. Colonna G, Pilati T, Rusconi F, Zecchi G, Dyes and Pigments, 2007, 75, 125 DPPs as Functional Dyes8 Fluorescence imaging Electroluminescence Solar cells Conductive polymers Photoconductive materials Two photon absorption Ions and molecules fluorescent sernsors Laser dyes Optical data storage Liquid crystals Electrochromic materials Field effect transistors 8. High Performance Pigments, Faulkner E B, Schwartz R J, WILEY-VCH, Weinheim, 2009, p.191 Examples – Two photon absorption Er Qian Guo et al9 9. Guo E Q, Ren P H, Zhang Y L, Zhang H C, Yang W J, Chem. Commun., 2009, 2859 Examples – Two photon absorption Er Qian Guo et al9 Compound λabs/nm λem/nm Δν/nm DPP-R DPP-DPA DPP-TPA DPP-R O C8H17 546 604 595 Br DPP-DPA N N Br 476 539 508 O O 70 65 87 Ph 2N 0,67 0,37 0,46 C8H17 λ2PA/nm δmax/GM δmax/MW 730 810 820 NPh 2 N N C8H17 Φ O C8H17 C8H17 DPP-TPA O N NPh 2 Ph 2N N O H17C8 9. Guo E Q, Ren P H, Zhang Y L, Zhang H C, Yang W J, Chem. Commun., 2009, 2859 110 1200 930 0,31 2,98 1,68 Examples – pH indicator Takuya Yamagata et al11 Cl Cl H N O N O O BnBr, K2CO3 DMF HN NH Bn Pd(OAc)2/SPhos tBuONa, PhMe Bn N N N Bn 120 oC O O O 100 oC N Bn O O 2 Cl Cl 2 2 + HCOOH 11. Yamagata T, Kuwabara J, Kanbara T, Tetrahedron Lett., 2010, 51, 1596 2 + TFA N O Examples – pH indicator Takuya Yamagata et al11 0 – 1000 eq of TFA 2 in CHCl3 (2 · 10-5 M) 1250 – 5000 eq of TFA 11. Yamagata T, Kuwabara J, Kanbara T, Tetrahedron Lett., 2010, 51, 1596 Examples – F- chemosensor Yi Qu et al12 C4H9 C4H9 O O N N Ar Ar FAr Ar N H N O O H F Compound 1 -Ar Br 2 3 12. Qu Y, Hua J, Tian H, Org. Lett., 2010, 12, 3320 Examples – NIR dyes Georg M. Fisher et al13,14, 15, 16 POCl3 ε = 125 000 – 261 000 M-1 cm-1 λabs = 684 – 864 nm λem = 708 – 881 nm ΦF = 0,32 – 0,69 13. Fisher G M, Jüngst Ch, Isomäki-Krondahl M, Gauss D, Möller H M, Daltrozzo E, Zumbusch A, Chem. Comm., 2010, 46, 5289 14. Fischer G M, Ehlers A P, Zumbusch A, Daltrozzo E, Angew. Chem. Int. Ed., 2007, 46, 3750 15. Fischer G M, Daltrozzo E, Zumbusch A, Angew. Chem. Int. Ed., 2011, 50, 1406 16. Fischer G M, Isomäki-Krondahl M, Göttker-Schnetmann I, Daltrozzo E, Zumbusch A, Chem. Eur. J., 2009, 15, 4857 Examples – NIR dyes Georg M. Fisher et al13,14, 15, 16 13. Fisher G M, Jüngst Ch, Isomäki-Krondahl M, Gauss D, Möller H M, Daltrozzo E, Zumbusch A, Chem. Comm., 2010, 46, 5289 14. Fischer G M, Ehlers A P, Zumbusch A, Daltrozzo E, Angew. Chem. Int. Ed., 2007, 46, 3750 15. Fischer G M, Daltrozzo E, Zumbusch A, Angew. Chem. Int. Ed., 2011, 50, 1406 16. Fischer G M, Isomäki-Krondahl M, Göttker-Schnetmann I, Daltrozzo E, Zumbusch A, Chem. Eur. J., 2009, 15, 4857 Examples – Solar cells Prashant Sonar et al17 Electrochemical bangap: 1,63 – 1,74 eV 1% power conversion for TFPDPP 17. Prashant S, Ging-Meng N, Ting Ting L, Ananth D, Zhi-Kuan C, J. Mater. Chem., 2010, 20, 3626 The End