Diapositiva 1 - Università degli Studi di Milano
advertisement
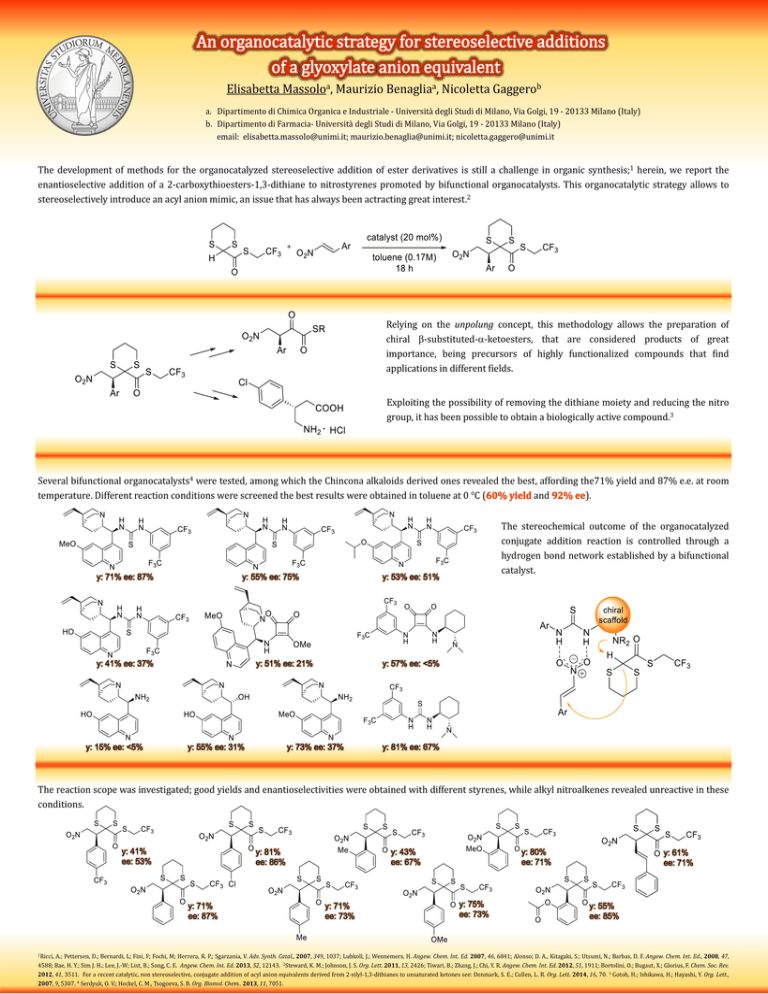
Elisabetta Massoloa, Maurizio Benagliaa, Nicoletta Gaggerob a. Dipartimento di Chimica Organica e Industriale - Università degli Studi di Milano, Via Golgi, 19 - 20133 Milano (Italy) b. Dipartimento di Farmacia- Università degli Studi di Milano, Via Golgi, 19 - 20133 Milano (Italy) email: elisabetta.massolo@unimi.it; maurizio.benaglia@unimi.it; nicoletta.gaggero@unimi.it The development of methods for the organocatalyzed stereoselective addition of ester derivatives is still a challenge in organic synthesis;1 herein, we report the enantioselective addition of a 2-carboxythioesters-1,3-dithiane to nitrostyrenes promoted by bifunctional organocatalysts. This organocatalytic strategy allows to stereoselectively introduce an acyl anion mimic, an issue that has always been actracting great interest.2 Relying on the unpolung concept, this methodology allows the preparation of chiral -substituted--ketoesters, that are considered products of great importance, being precursors of highly functionalized compounds that find applications in different fields. Exploiting the possibility of removing the dithiane moiety and reducing the nitro group, it has been possible to obtain a biologically active compound.3 Several bifunctional organocatalysts4 were tested, among which the Chincona alkaloids derived ones revealed the best, affording the71% yield and 87% e.e. at room temperature. Different reaction conditions were screened the best results were obtained in toluene at 0 °C ( and ). y: 71% ee: 87% y: 55% ee: 75% y: 41% ee: 37% y: 15% ee: <5% y: 53% ee: 51% y: 51% ee: 21% y: 55% ee: 31% The stereochemical outcome of the organocatalyzed conjugate addition reaction is controlled through a hydrogen bond network established by a bifunctional catalyst. y: 57% ee: <5% y: 73% ee: 37% y: 81% ee: 67% The reaction scope was investigated; good yields and enantioselectivities were obtained with different styrenes, while alkyl nitroalkenes revealed unreactive in these conditions. y: 41% ee: 53% y: 81% ee: 86% y: 71% ee: 87% 1Ricci, y: 43% ee: 67% y: 71% ee: 73% y: 80% ee: 71% y: 75% ee: 73% y: 61% ee: 71% y: 55% ee: 85% A.; Pettersen, D.; Bernardi, L; Fini, F; Fochi, M; Herrera, R. P.; Sgarzania, V. Adv. Synth. Catal., 2007, 349, 1037; Lubkoll, J.; Wennemers, H. Angew. Chem. Int. Ed. 2007, 46, 6841; Alonso; D. A., Kitagaki, S.; Utsumi, N.; Barbas, D. F. Angew. Chem. Int. Ed., 2008, 47, 4588; Bae, H. Y.; Sim J. H.; Lee, J.-W; List, B.; Song, C. E. Angew. Chem. Int. Ed. 2013, 52, 12143. 2Steward, K. M.; Johnson, J. S. Org. Lett. 2011, 13, 2426; Tiwari, B.; Zhang, J.; Chi, Y. R. Angew. Chem. Int. Ed. 2012, 51, 1911; Bortolini, O.; Bugaut, X.; Glorius, F. Chem. Soc. Rev. 2012, 41, 3511. For a recent catalytic, non stereoselective, conjugate addition of acyl anion equivalents derived from 2-silyl-1,3-dithianes to unsaturated ketones see: Denmark, S. E.; Cullen, L. R. Org. Lett. 2014, 16, 70. 3 Gotoh, H.; Ishikawa, H.; Hayashi, Y. Org. Lett., 2007, 9, 5307. 4 Serdyuk, O. V.; Heckel, C. M., Tsogoeva, S. B. Org. Biomol. Chem.. 2013, 11, 7051.







![Cooper_Abstract_MOF_2014[1]](http://s3.studylib.net/store/data/006662442_1-973d7b3fb19ef7da02e38c87851e45c2-300x300.png)