Physical & Chemical Properties/Changes Notes
advertisement

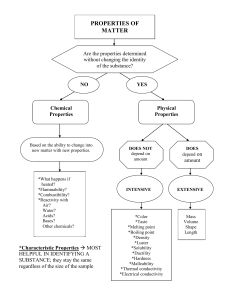
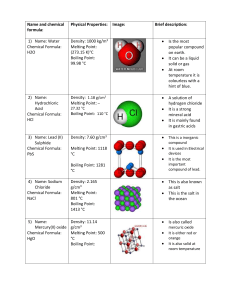
Physical & Chemical Properties Scientists use characteristic properties to identify matter Physical Properties Many physical properties can be observed or measured Mass, volume, density (mass/volume) Color, shape, odor, texture Melting, boiling point Strength, hardness, Magnetism Ability to conduct electricity or heat State of matter (solid, liquid, gas) Specific Temperatures of Phase Changes Substance Water Gold Carbon Mercury Nitrogen Oxygen NaCl Melting Pt oC 0 1063 3550 -39 -209 -218 801 Boiling Pt oC 100 2970 4827 357 -196 -183 1413 Density All matter has mass, volume & density Density determines whether an object will float or sink Density is the amount of mass in a volume Density = mass/volume D=m/V Density Examples Substance Air Helium Water Ice Steel Iron Chemical Formula Density in g/cm3 mixture He H20 H20 mixture Fe 0.00129 0.00018 1.0 0.92 7.8 7.86 Physical properties help to determine uses Copper used in electrical power lines Antifreeze (ethylene glycol) remains a liquid at temperature that would freeze or oil water in a car radiator Aluminum foil is lightweight, yet durable, water resistant and flexible Chemical Properties Describes how a substance reacts Reactivity – the ability of a substance to combine chemically with another substance (oxygen, acid, water or other substances) Iron reacts with oxygen to make rust Fe2O3 Flammability – the ability of a substance to react in the presence of oxygen and burn when exposed to a flame Wood is flammable Gold is nonflammable Comparisons Substance Physical Chemical Helium less dense air nonflammable Wood grainy texture flammable Baking Soda white powder reacts w/vinegar Rubbing alcohol clear liquid flammable Iron malleable reacts with oxygen Physical Change A change of matter from one form to another without a change in chemical properties Torn paper Melting ice Crushing a can Sanding wood Dissolving sugar Although a physical change takes place, a substance will maintain its chemical properties Melting, freezing and evaporation - all changes of state - are physical changes because the identity of the substance does not change ice, water, steam – all are water! Physical changes are often easily reversed. Mixtures can be separated using physical and chemical properties. Physical properties such as solubility, magnetism, density and size can help separate mixtures. Chemical change A change that occurs when a substance changes composition by forming one or more new substances. Chemical changes are always accompanied by physical changes. Evidence of Chemical Reaction Chemical change or “reactivity” results in Temperature change (always) Flame or light or explosion Change color Bubbling (gasses) [NOT BOILING] Oxidation (rusting or tarnishing) Solid formation (precipitation) [NOT FREEZING]