Chapter 3 Review ms science
advertisement

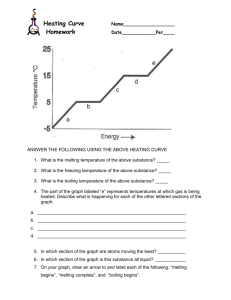
Chapter 3 Review MS Science Which of the following is an example of a physical change? • • • • A. B. C. D. Tarnishing Rusting Burning Melting melting Which of the following is a sign that a chemical change has occurred? • • • • A. B. C. D. Smoke Broken pieces Change in shape Change in state smoke When iron reacts with oxygen, what substance is produced? • • • • A. B. C. D. Tarnish Rust Patina Ashes rust What statement describes the physical property of density? • A. The distance between two points • B. How light is reflected from an object’s surface • C. The amount of mass in a given volume • D. The amount of space an object takes up the amount of mass in a given volume Which of the choices below describes a boiling point? • • • • A. B. C. D. A chemical property A chemical change A physical property A color change a physical property Which property is described by the ability of metals to be hammered into sheets? • • • • A. B. C. D. Mass Density Volume Malleability malleability Which of these is a chemical property? • • • • A. B. C. D. Size Density Flammability Volume flammability Which describes what volume is? • • • • A. B. C. D. The area of a square The amount of space an object takes up The distance between two points The temperature at which boiling begins the amount of space an object takes up What kind of change results in a new substance being produced? • • • • A. B. C. D. Chemical Mass Physical Change of state chemical What is conserved during any change? • • • • A. B. C. D. Color Volume Identity Mass mass A change in the identity of a substance that cannot be reversed by physical means is an example of a __? • • • • A. B. C. D. Physical change Chemical change Phase change None of the above chemical change _____ is anything that has mass and takes up space. • • • • A. B. C. D. Volume Density Length Matter matter The temperature at which a substance changes from a liquid to a gas is called the ________. • • • • A. B. C. D. Melting point Boiling point Freezing point Gas point boiling point A characteristic that determines how a substance will react is called a _____. • • • • A. B. C. D. Chemical property Flammability Physical property Phase change chemical property A characteristic that can be observed and measured is called a ________> A. B. C. D. Chemical property Flammability Physical property Mass physical property _____ is defined as mass per unit volume. • • • • A. B. C. D. Mass Density Volume Grams density . A process that does not change the identity of a substance is called a ________________________. • • • • A. B. C. D. Physical property Chemical property Physical change Chemical change physical change Solid, liquid, gas and plasma are the four ___________. • • • • A. B. C. D. States of chemicals States of matter The United States Mass states states of matter The temperature at which a solid becomes a liquid is called the ______ • • • • A. B. C. D. Melting point Boiling point Freezing point Condensation point melting point The Law that states that matter cannot be created or destroyed is the _____________. • • • • A. B. C. D. Law of mass Law of conservation of energy Law of states Law of Conservation of Mass Law of Conservation of Mass An object that sinks when placed in a beaker of water is _______ water. • • • • A. B. C. D. More dense than Has the same density of Less dense than None of the above more dense than What is conserved during any change? • • • • A. B. C. D. Volume Shape Mass Temperature Mass • Find the density of an object that has a mass of 60.0 g and a volume of 20.0 ml. Density = mass / volume Density = 60.0g / 20.0 ml = 3 g/ml Classify as either a chemical or physical change. Wood burning chemical Popsicle melting physical Baking a cake chemical Breaking an egg physical Adding sugar to your lemonade physical