Colorimetry
advertisement

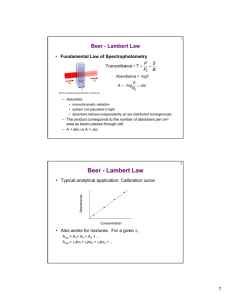
ENVE 201 Environmental Engineering Chemistry 1 COLORIMETRY Dr. Aslıhan Kerç Colorimetry • Colorimetric methods are applicable to dilute solutions. • For a colorimetric method to be quantitative, it must form a coumpound with definite color characteristics. • Color amount must be directly proportional to the concentration. • Colored compound must obey Beer’s Law and Lambert’s Law. Lambert’s Law • Relates the absorption of light to the depth or thickness of the colored liquid. • Each layer of equal thickness absorns an equal fraction of the light which traverses it. • Monochromatic light? Lambert’s Law I Io Cell length l • Intensity decreases exponentially with length. • This law is valid for homogeneous solutions Lambert’s Law I k 'l T 10 Io I Io Cell length l Io A log k ' l I l: length of absorbing layer T: transmittance of solution A: Absorbance or optical density k’ : constant for particular solution Beer’s Law Intensity of a ray of monochromatic light decreases expponentially as the concentration of the absorbing medium increases. I k ''C T 10 Io Io A log k ' ' C I k’’ : constant for particular solution C: concentration Beer’s Law If light is absorbed exponentially with concentration, the colored material is said to confirm Beer’s Law. Prepare a series of samples in desired concentration range, measure light transmission. If it forms a straight line on a semi-log paper obeys Beer’s law Lambert – Beer Law Two absorbtion laws are combined: I kCl T 10 Io Io A log kCl I Spectrophotometer Spectrophotometer Colorimeter - Spectrophotometer