Phys 12
advertisement

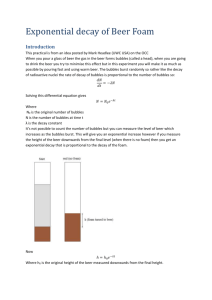
Phys 12 Half-Life of Beer Foam Purpose: To measure the half-life of non-alcoholic beer foam. Intro: We have used the equation 1 A A0 2 t T1 / 2 to model radioactive decay. We can measure the half-life of a sample by measuring the amount of nuclei available to decay at certain times. Plotting a graph of A vs t produces the standard decay curve. This can be t straightened by plotting log 0.5 A log 0.5 A0 . We will assume that the volume of T1 2 beer foam is proportional to the number of beer foam molecules. We will also assume that the volume of beer is constant and equals the sum of the volume of beer foam and the volume of liquid beer. Since it is difficult to measure the volume of beer foam, we will measure the volume of liquid beer. This will increase as the beer foam decreases. The change in liquid beer will equal the (opposite) change in beer foam. Procedure: 1) Place a strip of masking tape on the side of a large graduated cylinder. 2) Quickly pour a bottle of non-alcoholic beer into the graduated cylinder. 3) Mark off the initial height of the liquid beer. 4) Mark off the height of the liquid beer every 5 seconds until there is not a lot of change in the value. After that, take a reading every 2 or 3 minutes. 5) Record the maximum liquid beer height. 6) Calculate the volume of beer foam for each measurement of liquid beer height. Record this in a data table. 7) Plot the necessary graphs and calculate the half-life of beer foam.