File
advertisement

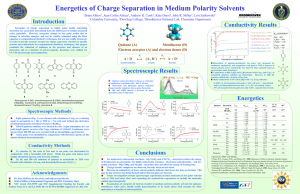
Exp 4A: Conductivity Of Aqueous Solutions • Purpose – Study conductivity of a series of solutions to determine the difference between strong electrolytes, weak electrolytes and nonelectrolytes – Use conductivity to distinguish between strong and weak acids and strong and weak bases – Use conductivity to study the effects of ion concentration • Conductivity of Solutions The conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct or allow the passage of electricity. • Conductivity units The SI unit of conductivity is siemens per meter (S/m) • Conductivity vs Conductance Consider a piece of wire. • • Electrical conductivity is a property of the material of the wire, and its value changes only with temperature (it decreases linearly with temperature). On the other hand conductance is the measure of the ease with which current can flow in the wire. It depends on the physical parameters of the wire (length,area of cross section) as well as the conductivity of the material of the wire. conductance=conductivity x area of cross section / length • • APPLICATIONS and CONCEPT CONNECTIONS Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems. For water quality- Conductivity is linked directly to the total dissolved solids (T.D.S.). High quality deionized water has a conductivity of about 5.5 μS/m, Typical drinking water in the range of 5-50 mS/m Sea water about 5 S/m (one million times higher than deionized water). • • • Conductivity is traditionally determined by measuring the AC (alternating currect) resistance of the solution between two electrodes. Dilute solutions follow Kohlrausch's Laws of concentration dependence and additivity of ionic contributions. Onsager gave a theoretical explanation of Kohlrausch's law by extending the Debye–Hückel theory. Exp 4A: Conductivity Of Aqueous Solutions • Electrolytes – Aqueous solutions of ionic compounds • Ionic compounds dissolve and dissociate in water NaCl(s) Na+(aq) + Cl-(aq) • Formation of positive and negative ions in solution • Solution conducts electricity • Strong electrolytes conduct electricity easily – Strong electrolytes are completely dissociated (100%) • Weak electrolytes conduct electricity poorly – Weak electrolytes are only partially dissociates (<100%) – Mostly undissociated = molecular form CH3COOH(l) H+(aq) + CH3COO-(aq) • Nonelectrolytes • Compounds that do not conduct electricity in solution • Compounds that do not form ions in aqueous solution Exp 4A: Conductivity Of Aqueous Solutions • NaCl(s) + H2O(l) Na+(aq) + Cl-(aq) – 1 positive charge + 1 negative charge • 2 ionic charges • MgCl2(s) + H2O(l) Mg2+(aq) + 2 Cl-(aq) – 1 ion with +2 charge, 2 ions with –1 charge • 4 ionic charges • Fe(NO3)3+ H2O(l) Fe3+(aq) + 3 NO3-(aq) – 1 ion with +3 charge, 3 ions with –1 charge • 6 ionic charges • Conductivity depends on – Concentration of ions – Charge of ions – (Size of ions (mobility in solution: large ions move more slowly)) Exp 4A: Conductivity Of Aqueous Solutions Electrolytes conduct electricity http://nvcicourse.accd.edu:8900/SCRIPT/1020720051/scripts/student/serve_page.pl/1020720051/chapter4/animations.html?1118762248+1859016556+OFF+olc/dl/1 71024/4_1_Stg_Wk_Nonelelytes.swf+ Exp 4A: Conductivity Of Aqueous Solutions • Strong electrolytes – Conduct electricity easily – Electrolyte (almost) completely dissociated in solution NaCl(s) + H2O(l) Na+(aq) + Cl-(aq) • Weak electrolytes – Poorly conducting solutions – Electrolytes mostly in molecular form with few ions NaH2PO4(s) + H2O(l) Na+(aq) + H2PO4-(aq) • Magnitude of conductance of a solution – proportional to the number and type of ions in solution • More ions (higher ion concentration): more conductivity • More ionic charges: more conductivity Exp 4A: Conductivity Of Aqueous Solutions • Strong Acids – HCl(g) + H2O(l) H+(aq) + Cl-(aq) – HNO3(l) + H2O(l) H+(aq) + NO3-(aq) • Strong Bases – KOH(s) + H2O(l) K+(aq) + OH-(aq) • Weak Acids – CH3COOH(l) + H2O(l) H+(aq) + CH3COO-(aq) • Weak Bases – NH3(g) + H2O(l) NH4+(aq) + OH-(aq) Exp 4A: Conductivity Of Aqueous Solutions • Strong and Weak electrolytes Strong Electrolyte Weak Electrolyte Exp 4A: Conductivity Of Aqueous Solutions • Mixtures of electrolytes – Conductance shows additive effect if electrolytes do not react with each other • more ions, more charges, more conductivity – If a chemical reaction occurs between the electrolytes, the properties of the new substance(s) determine conductivity • typically reaction between a weak acid and a base or a weak base and an acid NH3(aq) + HCl(aq) NH4+(aq) + Cl-(aq) Exp 4A: Conductivity Of Aqueous Solutions Dilution • A certain amount of a solution added to an amount of solvent to lower the concentration – – – Add 10 mL of 1.0 M NaCl to 90 mL of H2O Final volume = 10 mL + 90 mL = 100 mL • • Dilution = 1:10 Concentration = (10 mL x 1.0 M NaCl)/ 100 mL = 0.10 M NaCl • The concentration changes. Does the total amount of NaCl particles change? Dilution formula: • initial molarity (mol/L) x initial volume (L) = final molarity (mol/L) x final volume (L) = Mi x V i = M f x V f = mol/L x L = mol Exp 4A: Conductivity Of Aqueous Solutions Prelab Question 4a • How will you prepare 10 mL of 0.050 M HCl from 0.10 M HCl? Answer • Use dilution formula V1 x M1 = V2 x M2 – – – – • M1 = 0.10 M M2 = 0.050 M V2 = 10 mL V1 = V2 x M2/M1 = 10 mL x 0.050 M/0.10M = 5.0 mL of 0.10 M HCl Take 5.0 mL of 0.10 M hydrochloric acid in a 10.0-mL graduated cylinder and dilute to 10 mL with dH2O Exp 4A: Conductivity Of Aqueous Solutions Prelab Question 5a • How will you prepare 80 mL of 0.10 M CH3COOH from 6.0 M acetic acid? Answer • Use dilution formula V1 x M1 = V2 x M2 – – – – • M1 = 6.0 M M2 = 0.10 M V2 = 80 mL V1 = V2 x M2/M1 = 80 mL x 0.10 M/6.0M = 1.3 mL of 6.0 M acetic acid Take 1.3 mL of 6.0 M acetic acid in a 5.0-mL graduated cylinder or use a 5.0 mL pipet. Add ~ 50 mL dH2O to a 100-mL graduated cylinder, then add the 1.3 mL of acetic acid. If you use a 5.0 mL cylinder for the acetic acid, rinse it out with dH2O and add it to the 100-mL cylinder. Repeat this process 2 more times. Add dH2O to a total volume of 80 mL. Prelab Question 5b • Same as 5a Exp 4A: Conductivity Of Aqueous Solutions Part 1: Compare conductance of • • 20 mL dH2O, 20 mL tap water, 20 mL ethanol in 50 mL beaker Why is there a difference in conductance, if any, between distilled water and tap water? Part 2: Measure conductance of hydrochloric acid solutions • • • • 20 mL 0.10 M HCl 0.050 M HCl: dilute 10 mL 0.10 M HCl + 10 mL dH2O 0.020 M HCl: dilute 10 mL 0.050 M HCl + 15 mL dH2O How do you make dilutions? See prelab assignment 4! Exp 4A: Conductivity Of Aqueous Solutions Part 3: Measure conductivity in different solutions Solution Concentration 1 10 mL 0.10 M HNO3 + 10 mL dH2O 2 10 mL 0.10 M KOH + 10 mL dH2O 3 10 mL 0.10 M KCl + 10 mL dH2O 4 10 mL 0.10 M KNO3 + 10 mL dH2O 5 10 mL 0.10 M Ca(NO3)2 + 10 mL dH2O 6 10 mL 0.10 M NH3 + 10 mL dH2O 7 10 mL 0.10 M HC2H3O2 + 10 mL dH2O 8 10 mL 0.10 M HCl + 10 mL 0.10 M KNO3 Calculate new concentrations 9 10 mL 0.10 M HNO3 + 10 mL 0.10 M KCl Calculate new concentrations 10 11 10 mL 0.10 M HCl + 10 mL 0.10 M KOH 10 mL 0.10 M NH3 + 10 mL 0.10 M HC2H3O2 Calculate new concentrations Calculate new concentrations Conductance Exp 4A: Conductivity Of Aqueous Solutions Measure conductivity in different solutions Solution Concentration 1 10 mL 0.10 M HNO3 + 10 mL dH2O 0.05 2 10 mL 0.10 M KOH + 10 mL dH2O 0.05 3 10 mL 0.10 M KCl + 10 mL dH2O 0.05 4 10 mL 0.10 M KNO3 + 10 mL dH2O 0.05 5 10 mL 0.10 M Ca(NO3)2 + 10 mL dH2O 0.05 6 10 mL 0.10 M NH3 + 10 mL dH2O 7 10 mL 0.10 M HC2H3O2 + 10 mL dH2O 8 10 mL 0.10 M HCl + 10 mL 0.10 M KNO3 9 10 mL 0.10 M HNO3 + 10 mL 0.10 M KCl 10 11 10 mL 0.10 M HCl + 10 mL 0.10 M KOH 10 mL 0.10 M NH3 + 10 mL 0.10 M HC2H3O2 Conductance Exp 4A: Conductivity Of Aqueous Solutions Next week: • • • No Formal report. Turn in the following, stapled in this order • On paper: Type or write 1)a summary of the experiment and 2)your conclusions about the relationship between conductivity and concentration • Data and Calculations sheets for Exp 4A: Conductivity Of Aqueous Solutions • Answers to post-lab questions on the lab manual sheets Prelab Assignment for Exp 4B: Ionic Reactions in Aqueous Solutions – Avoid getting definitions from Google or Wikipedia. Use a textbook or lab manual or a science dictionary – Read Prelab preparations and protocol – Answer Prelab questions 1a-d, 2a-h, 3, 4