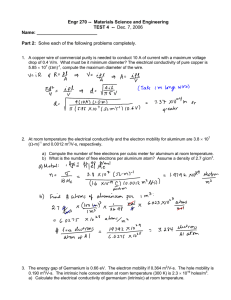
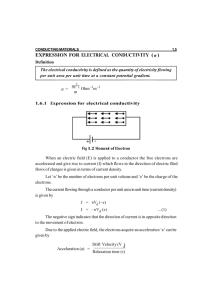
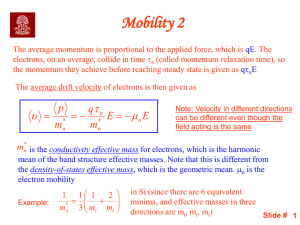
(a) Compute the electrical conductivity of a 5.1-mm
advertisement
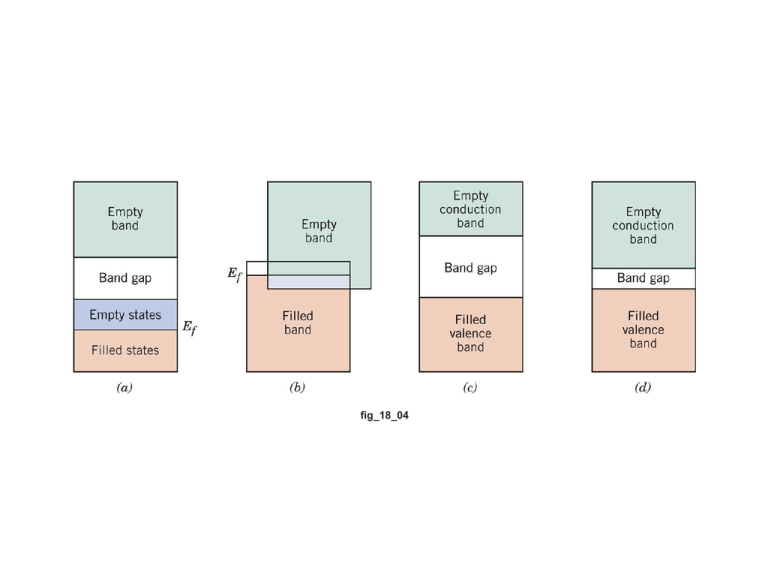
fig_18_04 18.1 (a) Compute the electrical conductivity of a 5.1-mm diameter cylindrical silicon specimen 51 mm long in which a current of 0.1 A passes in an axial direction. A voltage of 12.5 V is measured across two probes that are separated by 38 mm. (b) Compute the resistance over the entire 51 mm of the specimen. = = 1 Il = = VA Il d 2 V 2 (0.1 A)(38 103 m) -1 = 14.9 ( m) 5.1 103 m 2 (12.5 V)() 2 l l R= = A A l 2 d 2 51 103 m = 14.9 ( m)1 2 5.1 103 m () 2 = 168 18.10 (a) Calculate the drift velocity of electrons in germanium at room temperature and when the magnitude of the electric field is 1000 V/m. (b) Under these circumstances, how long does it take an electron to traverse a 25-mm length of crystal? (mobility of electrons in Ge is 0.38 m2/v-s) vd = e E = (0.38 m2 /V- s)(1000 V/m) = 380 m/s l 25 103 m t = = = 6.6 10-5 s vd 380 m/s 18.11 At room temperature the electrical conductivity and the electron mobility for copper are 6.0 107 (ohm-m)-1 and 0.0030 m2/V-s, respectively. (a) Compute the number of free electrons per cubic meter for copper at room temperature. (b) What is the number of free electrons per copper atom? Assume a density of 8.9 g/cm3. 6.0 107 ( m)1 n= = 1.25 1029 m-3 = e e (1.602 1019 C)(0.003 m2/V- s) (b) In order to calculate the number of free electrons per copper atom, we must first determine the number of copper atoms per cubic meter, NCu. From Equation 4.2 using the atomic weight value for Cu found inside the front cover—viz. 63.55 (and g/mol) N Cu = N A ACu ( 6.022 1023 atoms/ mol)(8.9 g/cm3)(106 cm3 / m3) = 63.55 g/mol = 8.43 1028 m-3 And, finally, the number of free electrons per aluminum atom is just n/NCu 1.25 1029 m3 = = 1.48 28 3 N Cul 8.43 10 m n Intrinsic Semiconduction 18.18 (a) Using the data presented in Figure 18.16, determine the number of free electrons per atom for intrinsic germanium and silicon at room temperature (298 K). The densities for Ge and Si are 5.32 and 2.33 g/cm3, respectively. (b) Now explain the difference in these free-electron-per-atom values. table_18_03 Extrinsic Semiconduction *18.21 At room temperature the electrical conductivity of PbTe (Lead telluride) is 500 (Ω-m)–1, whereas the electron and hole mobilities are 0.16 and 0.075 m2/V-s, respectively. Compute the intrinsic carrier concentration for PbTe at room temperature. *18.25 An n-type semiconductor is known to have an electron concentration of 3 1018 m-3. If the electron drift velocity is 100 m/s in an electric field of 500 V/m, calculate the conductivity of this material. 18.30 Germanium to which 5 1022 m-3 Sb atoms have been added is an extrinsic semiconductor at room temperature, and virtually all the Sb atoms may be thought of as being ionized (i.e., one charge carrier exists for each Sb atom). (a) Is this material ntype or p-type? (b) Calculate the electrical conductivity of this material, assuming electron and hole mobilities of 0.1 and 0.05 m2/V-s, respectively. = n| e |e = (5 10 22 m-3 )(1.602 10-19 C)(0.1 m2 /V- s) = 800 (-m)-1 The Temperature Dependence of Carrier Concentration • 18.32 Calculate the conductivity of intrinsic silicon at 100°C. Intrinsic Semiconductors: Conductivity vs T • Data for Pure Silicon: -- increases with T -- opposite to metals ni e e h E gap / kT ni e material Si Ge GaP CdS band gap (eV) 1.11 0.67 2.25 2.40 Selected values from Table 18.3, Callister & Rethwisch 8e. Adapted from Fig. 18.16, Callister & Rethwisch 8e. 10 Factors That Affect Carrier Mobility • 18.38 Calculate the room-temperature electrical conductivity of silicon that has been doped with 2 × 1023 m–3 of arsenic atoms. n | e | e (2 1023 m3)(1.602 1019 C)(0.05 m2 / V s) 1600 ( m)1