Patrick Steel
advertisement

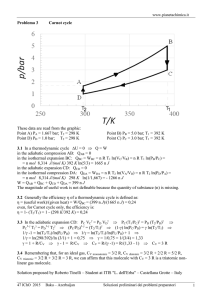
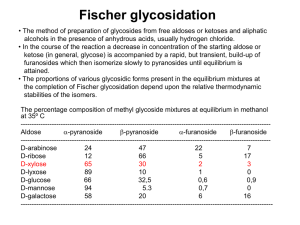
∂ Efficient Catalytic Syntheses of Organoboronate Esters Ubiquitous building blocks for the fine chemical and pharmaceutical industries ∂ Chemistry Initiative NORSC Sustainable Ramside Hall, Durham 24th April 2012 Patrick Steel Department of Chemistry, Durham University, UK p.g.steel@durham.ac.uk Why make aryl boronates? ∂ Hall, Boronic Acids, Wiley-VCH, 2nd Ed 2011 Why make aryl boronates? • Non-toxic • Tolerant to air and water • Stable in the absence of a catalyst ∂ • React under mild conditions • Reaction by-product is inert and benign to humans Hall, Boronic Acids, Wiley-VCH, 2nd Ed 2011 Classical Aryl Boronate Synthesis a Sustainable Chemistry Challenge? F H2N Br 1. i. n-BuLi (2 eq.), THF, 0 °C ii. TMSCl 2. i. t-BuLi (2.2 eq.), Et2O, -78 °C ii. B(OMe)3 (xs), -78 °C iii. 0.1N aq HCl F H2N B(OH)2 45 % Asher et al., Tetrahedron Lett., 2003, 44, 7719 ∂ NEt2 O MeO O NEt2 i. s-BuLi, TMEDA, THF, -78 °C ii. B(OMe)3 O MeO B(OH)2 iii. 10 % aq. HCl 88 % Snieckus et al., J. Org. Chem., 2009, 74, 4094 O Functional Group Tolerant Aryl Boronate Synthesis Pd(dppf)Cl2 (3 mol%), KOAc (3 eq.), B2pin2 (1.1 eq.) O O O Br B DMSO, 80 °C, 1 h O 80 % Ishiyama, Miyaura et al., J. Org. Chem., 1995, 60, 7508 ∂ CuI (10 mol%), nBu3P (13 mol%), KOtBu (1.5 eq.), B2pin2 (1.5 eq.) O Br B O THF, 25 °C, 24 h 69 % Kleeberg, Marder et al., Angew. Chem. Int. Ed., 2009, 48, 5350 Iridium Catalysed Arene C-H Borylation ∂ Mkhalid, Barnard, Marder, Murphy, Hartwig, Chem. Rev., 2010, 110, 890 Iridium Catalysed Arene C-H Borylation ∂ Mkhalid, Barnard, Marder, Murphy, Hartwig, Chem. Rev., 2010, 110, 890 Iridium Catalysed Borylation – An Atom Economical Process ∂ Iridium Catalysed Borylation – An Atom Economical Process ∂ Iridium Catalysed Borylation – An Atom Economical Process ∂ ‘One-pot’ Transformations from Arenes S i. [Ir(OMe)(cod)]2 (1.5 mol%), dtbpy (3 mol%) HBpin (1.5 eq) hexanes, 25 ˚C, 0.5h S CF3 ii. removal of volatiles iii. aq. K3PO4•nH2O, 4-BrC6H4CF3, Pd(PPh3)4 (2 mol%) DME, 80 ˚C, 8 h 85% Maleczka, Smith et al., Tetrahedron, 2008, 64, 6103 ∂ CO2Me i. [Ir(OMe)(cod)]2 (1.5 mol%), dtbpy (3 mol%) B2pin2 (0.5 eq) hexane (1 vol) 25 ˚C, 4h Cl Cl ii. aq. K3PO4; 4-BrC6H4CO2Me, PdCl2(dppf) (3 mol%) DMF (2 vol), 60 ˚C, 2 h Cl 96% Cl Ishiyama, Miyaura et al., Tetrahedron, 2008, 64, 4967 One-pot C-H Borylation/Suzuki-Miyaura Cross-Coupling O [Ir(OMe)(cod)]2 (1.5 mol%), dtbpy (3 mol%) B O B2pin2 (1 equiv), solvent, 80 ˚C, 6 h ∂ Solvent Temp ( ˚C) Time (h) GC-MS Conversion (%) DMF 80 6 0 1,4-dioxane 80 6 25 MeCN 80 6 37 2-MeTHF 80 6 85 MTBE 80 6 100 Peter Harrisson One-pot C-H Borylation/Suzuki-Miyaura Cross-Coupling CO2Me O [Ir(OMe)(cod)]2 (1.5 mol%) dtbpy (3 mol%) B Pd(dppf)Cl2 (3 mol%) KOH (5 eq) O B2pin2 (1 eq) MTBE 80 ˚C, 6 h 4-MeO2CC6H4I (1.2 eq) MTBE - H2O, 80 ˚C, 3 h ∂ Peter Harrisson 90% One-pot C-H Borylation/Suzuki-Miyaura Cross-Coupling CO2Me O [Ir(OMe)(cod)]2 (1.5 mol%) dtbpy (3 mol%) B Pd(dppf)Cl2 (3 mol%) KOH (5 eq) O B2pin2 (1 eq) MTBE 80 ˚C, 6 h 4-MeO2CC6H4I (1.2 eq) MTBE - H2O, 80 ˚C, 3 h ∂ Peter Harrisson 90% One-pot C-H Borylation/Suzuki-Miyaura Cross-Coupling CO2Me O [Ir(OMe)(cod)]2 (1.5 mol%) dtbpy (3 mol%) B Pd(dppf)Cl2 (3 mol%) KOH (5 eq) O B2pin2 (1 eq) MTBE 80 ˚C, 6 h 4-MeO2CC6H4I (1.2 eq) MTBE - H2O, 80 ˚C, 3 h ∂ Peter Harrisson 90% One-pot C-H Borylation/Suzuki-Miyaura Cross-Coupling ∂ Peter Harrisson Microwave Accelerated “One-Pot” Biaryl Synthesis ∂ Peter Harrisson Challenges for Ir-Catalysed Arene C-H Borylation Regiocontrol [Ir(OMe)(cod)]2 dtbpy R R Bpin B2pin2 80 ˚C R R Rbig R ∂ R R 2 Rsmall 1 Challenges for Ir-Catalysed Arene C-H Borylation Carboxylic Acid Vs. Boronic Acid: Heterocyclic Organoboronic Acids Chemical Space Comparison Green = Available Red = Non Available MeO Phenyl Aromatic Space Heterocyclic Ar Space CO2H ∂ N OH OH MeO B CO2H N B N OH OH N Carboxylic acids open up more heterocyclic design space! Data – Courtesy David Blakemore Pfizer - Neusentis Challenges for the Synthesis of Heterocyclic Boronate Esters 1 2 N H N H N TIPS N Bpin [Ir(OMe)(cod)]2 (2.5 mol%) dtbpy (5 mol%) B2pin2 (0.5 eq) N hexanes, 80 ˚C Bpin ∂ N + N 1:1 [Ir(OMe)(cod)]2 (1.5 mol%) dtbpy (3 mol%) B2pin2 (2 eq) N MTBE, mW, 80 ˚C Bpin Bpin Bpin N Bpin N 89% 1 : 1 Challenges for the Synthesis of Heterocyclic Boronate Esters 1 2 N H N H N TIPS N Bpin [Ir(OMe)(cod)]2 (2.5 mol%) dtbpy (5 mol%) B2pin2 (0.5 eq) N hexanes, 80 ˚C Bpin ∂ N + N 1:1 [Ir(OMe)(cod)]2 (1.5 mol%) dtbpy (3 mol%) B2pin2 (2 eq) N MTBE, mW, 80 ˚C Bpin Bpin Bpin N Bpin N 89% 1 : 1 Quinoline Boronate Esters ∂ Peter Harrisson Where Now for ‘Cleaner Greener’ Aromatic C-H Borylation? Other sequential chemistries Faster more effective Catalysts. Better ligand sets to control regiochemistry. ∂ Where Now for ‘Cleaner Greener’ Aromatic C-H Borylation? ∂ Where Now for ‘Cleaner Greener’ Aromatic C-H Borylation? Other sequential chemistries Faster more effective Catalysts. Better ligand sets to control regiochemistry. ∂ Cheaper metals than “Ir” – Cu???? Alkyl Boronate Esters via Cu-Catalysed C-X Activation ∂ Hazmi Tajuddin 2012 Durham Lectures Prof John Hartwig University of California, Berkeley ∂ Monday 14th May Tuesday 15th May Wednesday 16th May Department of Chemistry, Durham University All Lectures Commence at 4pm – Visitors Welcome For Further Details p.g.steel@durham.ac.uk Understanding and Optimising Chemical Processes 2nd & 3rd July 2012 Department of Chemistry Durham University This two-day programme provides delegates with an opportunity to augment their process knowledge using Principal Components Analysis (PCA) and Design of Experiments (DoE) methodologies to expand process understanding, establish and develop capability, ensure robust and in-control ∂ processes, improve quality and reduce operational and environmental costs. Keynote Speaker Martin Owen Product Development, GlaxoSmithKline, Stevenage Further Information & Registration Contact: Matthew Linsley, ISRU, Newcastle University matthew.linsley@newcastle.ac.uk 29 Acknowledgements Prof Todd Marder Peter Harrisson Hazmi Tajuddin Bianca Bitterlich Prof Lei Liu (Tsinghua University, Beijing) ∂ Alan Kenwright (NMR) Jackie Mosely (MS) Andrei Batsanov (X-ray) James Morris (Syngenta) Aoife Maxwell (GSK) Lena Shukla (GSK) Understanding and Optimising Chemical Processes 2nd & 3rd July 2012 Department of Chemistry Durham University This two-day programme provides delegates with an opportunity to augment their process knowledge using Principal Components Analysis (PCA) and Design of Experiments (DoE) methodologies to expand process understanding, establish and develop capability, ensure robust and in-control ∂ processes, improve quality and reduce operational and environmental costs. Keynote Speaker Martin Owen Product Development, GlaxoSmithKline, Stevenage Further Information & Registration Contact: Matthew Linsley, ISRU, Newcastle University matthew.linsley@newcastle.ac.uk 31 ∂