Glycosyl amines
advertisement
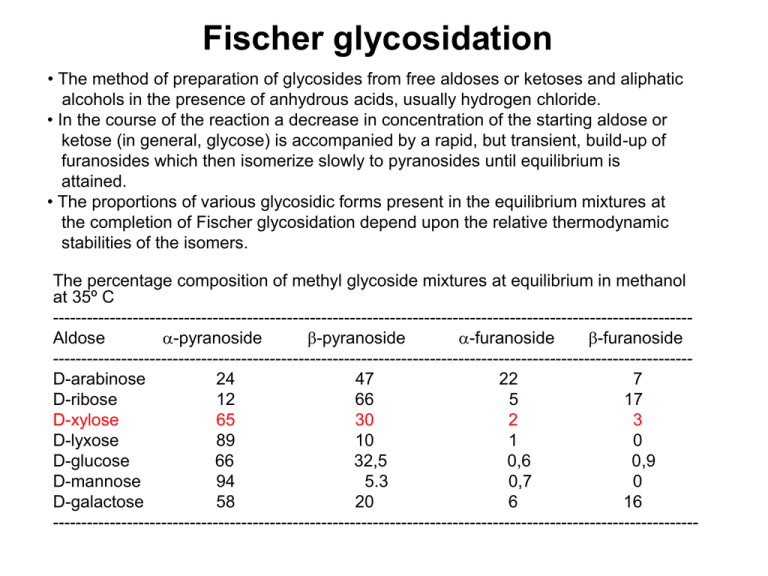
Fischer glycosidation • The method of preparation of glycosides from free aldoses or ketoses and aliphatic alcohols in the presence of anhydrous acids, usually hydrogen chloride. • In the course of the reaction a decrease in concentration of the starting aldose or ketose (in general, glycose) is accompanied by a rapid, but transient, build-up of furanosides which then isomerize slowly to pyranosides until equilibrium is attained. • The proportions of various glycosidic forms present in the equilibrium mixtures at the completion of Fischer glycosidation depend upon the relative thermodynamic stabilities of the isomers. The percentage composition of methyl glycoside mixtures at equilibrium in methanol at 35º C ---------------------------------------------------------------------------------------------------------------Aldose -pyranoside -pyranoside -furanoside -furanoside ---------------------------------------------------------------------------------------------------------------D-arabinose 24 47 22 7 D-ribose 12 66 5 17 D-xylose 65 30 2 3 D-lyxose 89 10 1 0 D-glucose 66 32,5 0,6 0,9 D-mannose 94 5.3 0,7 0 D-galactose 58 20 6 16 ----------------------------------------------------------------------------------------------------------------- OMe O OH HO HO O OH HO HO OMe (a) OMe HO HO O HO OH OH O (b) (e) HOOMe (d) (d) (a) (c) (b) (e) (d) (c) (e) (a) (b), (c) The time dependence of glycosidation of D-xylose (c) in 0,5 % HCl in methanol at 25 °C Source: Monosaccharides. Their Chemistry and Their Roles in Natural Products, P.M. Collins, R.J. Ferrier, Wiley, Chichester, 1995. HO HO HO HO O + HO OMe 30 % methyl α-D-xylopyranoside O methyl -D-xylopyranoside MeOH + HO OMe HO 65 % HO HO O OH D-xylose (α,β-D-xylopyranose) + H+ HO O OH + HO OMe O OH OMe OH 2% methyl α-D-xylofuranoside OH 3% methyl -D-xylofuranoside Equilibrium mixture of methyl D-xylosides originating from the Fischer glycosidation of D-xylose in methanolic solution of hydrogen chloride at 35º C. Methyl D-xylopyranoside is the major product, due to the anomeric effect, which is characteristic for the tetrahydropyran rings with an electronegative substituent in position 2. • Anomeric effect – a decrease of the stability of the equatorial anomer, due to the interaction of its electronegative substituent X with free electron pairs of the pyranose oxygen atom, which causes the relative increase of the stability of the axial anomer. This effect, for the first time observed in saccharides, is a general phenomenon of both cyclic and acyclic molecules containing 1,3-grouping of heteroatoms. O O X X OH HO HO OH O HOOMe 66 % methyl -D-glucopyranoside HO HO O OMe HO 32,5 % methyl -D-glucopyranoside Anomeric effect O O O O R R The simplest explanation of the effect is, that the equatorial position of the anomeric substituent has the dipoles of both heteroatoms partly parallel and thus repulsing. On the other side, its axial position has these dipoles approximately antiparallel, so that is representing a more stable and energetically less demanding structure. .. O .. O . . O O R R An alternative and more accepted explanation is that the axial position is stabilized by the conjugation between the axial free electron pair of the pyranose oxygen atom and the σ* orbital of the axial C-OR bond. Mechanism of the Fischer glycosidation (I) HO + H+ H O OH OH + - H 2O HO + H 2O OH + O OH H OH - H+ D-xyl - MeOH + MeOH - MeOH + MeOH - H+ + H+ HO OH OH OH - H 2O OMe OH HO O OH OMe + H2O OH Bolded route of transformation is more probable. Source: Monosaccharides. Their Chemistry and Their Roles in Natural Products, P.M. Collins, R.J. Ferrier, Wiley, Chichester, 1995. Mechanism of the Fischer glycosidation (II) HO O O OH OMe OMe OH OH OH OH + H+ (b) (a) (b,d) MeOH - H+ (H+) - MeOH (c) HO OH OH OMe OMe OH HO (d) OH OH + OMe + H+ (- MeOH) OH Bolded route of transformation is more probable. Source: Monosaccharides. Their Chemistry and Their Roles in Natural Products, P.M. Collins, R.J. Ferrier, Wiley, Chichester, 1995. Thermodynamic equilibria of the Fischer glycosidation of D-glucose, D-mannose and D-galactose OH D-gluko HO HO O HO HO HOOMe HO D-mano OH OH OMe 32,5 % OH OH HO HO OMe OH HO OH O OHHO HO OMe 5,3 % HO O D-galakto HO 58 % OMe 0,7 % 0% OH O OH O HO HOOMe O OH HO HO OMe 94 % OH 0,9 % OH OMe HO OH 0,6 % O HO OMe O OH HO HO 66 % O HO HO O OH O OH OMe HO 20 % HO OMe OH OH 6% O OH HO OH OMe OH 16 % Internal glycosides (anhydrides of saccharides) OH O H+ O OH O H2O (OH)3 (OH)3 D-Glc D-Gal D-Man D-Tal 0,2 % 0,8 % 0,8 % 2,8 % O O O 14 % O O O O D-All O D-Gul D-Alt D-Ido 65 % 65 % 86 % O O O O OH O O O O O O HOTs OH HO (OH)2 O O alebo DMF OH (OH)3 (OH)2 D-Glc D-Gal D-Man 35 % 87 % 22 % O D-Tal 86 % D-All 78 % H+ H2O D-Glc 0,2 % D-Gal 0,8 % D-Man 0,8 % D-Tal 2,8 % D-All 14 % D-Gul 65 % D-Alt 65 % D-Ido 86 % R = OH Generation of the internal glycosides in water (Reversion – generation of oligosaccharides in acidic aqueous solutions.) HOTs or DMF D-Glc 35 % D-Gal 87 % D-Man 22 % D-Tal 86 % D-All 78 % Generation of the internal glycosides in aprotic solvents Preparation of sugar dithioacetals OH OH OH SEt HO OH OH O EtSH OH OH OH konc. HCl HO OH SEt SEt HO OH D-xylose HO O OH SEt OH D-xylose diethyl dithioacetal HgCl 2 CH=O ( OAc )n OAc SO 2R RSO 2 SR RS CdCO 3 ( H2O O OAc )n ( Ac 2O OAc CH3COCH3 Py [O] RS ( ( OH )n OH OH )n-1 OH SR OH )n OH H2 CH3 H _ HO CH=O Ni ( OH )n-1 OH Sugar dithioacetals are being used for preparation of acyclic derivatives of sugars OH OH 1 % HCl/H2O, 20°C, 20 h OH OH OH OH OH OH SEt SEt HO HO O OH OH OH SEt SEt OH 1. HgO, 5 h, 2. 2. EtOH, HgO, HgCl2 OH OH OEt SEt HO OH O OH OEt OH OH OH Acyclic dithioacetals can also be used for preparation of foranoid derivatives of sugars. There is being applied the knowledge that the closure of the five membered rings is more rapid than that the closure of the six membered rings. Relative reaction rates at 50 °C (for eight-membered ring = 1) for reaction Br (CH2)n-2 COO (CH2)n-2 C O + Br O n = Ring size G. Illuminati, L. Mandolini, Acc. Chem. Res. 14, 95 (1981). The Nef type glycosidation of 1-deoxy-1-nitroalditols NO2 HO OH HO 1. NaOMe, MeOH HO OH OH O 2. HCl, MeOH, -30 °C HO OH OMe + OH OMe HO major (1,2-cis) OH O HO OH minor (1,2-trans) 14% 76% NaOMe MeOH + - H , - NOH, - H2O H NO2Na HO + H HO OH OH MeOH OH H HO + OH N OH HO HO OH H OH O HO HO + O Me H OH N OH OH OH M. Vojtech, M. Petrušová, B. Pribulová, L. Petruš, Tetrahedron Lett. 49 (2008) 3112–3116. The Nef type glycosidation of 1-deoxy-1-nitroalditols at -30°C M. Vojtech, M. Petrušová, B. Pribulová, L. Petruš, Tetrahedron Lett. 49 (2008) 3112–3116. _________________________________________________________________________ Nitrohexitol cis-Furanoside Yield (%) trans-Furanoside Yield (%) _________________________________________________________________________ NO2 OH OH HO O OMe O OMe HO HO OH 36 55 HO OH HO OH HO OH OH NO2 OH OH OH O OMe O OMe HO HO OH 11 78 HO OH HO OH HO OH OH NO2 OH OH OH O OMe O OMe HO HO HO 36 OH 54 OH H O OH HO OH OH NO2 OH OH HO O OMe O OMe HO HO HO 76 14 OH OH H O OH HO OH OH NO2 OH OH OH O OMe O OMe HO HO HO 65 25 HO OH H O OH HO OH OH NO2 OH OH HO O OMe O OMe HO HO HO 74 16 HO HO OH OH NO2 HO HO OH OH OH OH O OMe OH 67 24 OH OH HO OH HO OH OH NO2 OH OH OH O OMe O OMe HO H O OH 83 8 OH OH HO OH HO OH OH _________________________________________________________________________ HO O OMe HO Glycosyl amines Derivatives of sugars in which the glycosyl moiety is linked to a primary, secondary or a tertiary amino group. If two glycosyl moieties are linked to a secondary amino group, the derivatives are named as bisglycosyl amines. According to the non-saccharidic nature of the amino group, they are devided into unsubstituted, aliphatic and aromatic glycosyl amines. Aromatic glycosyl amines are much more stable than aliphatic ones. Similarly as free aldoses or ketoses (glycoses), they undergo mutarotation. A treatment with mineral acids causes their decomposition to glycose and amine or ammonia. A characteristic reaction of glycosyl amines is the Amadori reaction for which the best catalysts are strongly basic anions of weak acids. Amadori reaction anion of a weak acid (strong_ base) B n Glycosyl amine _ _ _ B _ n n n 1-amino-1-deoxy2-ketose • Amadori reaction - base-catalyzed isomerization of the aldose-derived glycosyl amines to 1-amino-1-deoxy-2ketoses. The reaction is similar to Lobry de BruynAlberda van Ekenstein reaction of aldoses. • The reaction stays at the beginning of the origin of Maillard melanoids, brown polymers produced by subsequent reactions of the products of the Amadori reaction, carbonyl compounds and amino acids. Thus the Maillard reactions also are responsible for the formation of brown products (melanoids) when foods containing carbohydrates and proteins are processed under heating http://brewery.org/library/Maillard_CS0497.html • Similar base-catalyzed isomerization of the 2-ketosederived glycosyl amines to 2-amino-2-deoxy-aldoses is called as the Heyns reaction. Melanin is a class of compounds found in the plant, animal, and protista kingdoms, where it serves predominantly as a pigment. The class of pigments are derivatives of the amino acid tyrosine. The increased production of melanin in human skin is called melanogenesis. It is stimulated by the DNA damages that are caused by UVB-radiation,[1] and it leads to a delayed development of a tan. This melanogenesis-based tan takes more time to develop, but it is long lasting.[2] http://en.wikipedia.org/wiki/Melanin Melamine is an organic base and a trimer of cyanamide, with a 1,3,5triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass and, if mixed with resins, has fire retardant properties due to its release of nitrogen gas when burned or charred, and has several other industrial uses. Melamine is also a metabolite of cyromazine, a pesticide. It is formed in the body of mammals who have ingested cyromazine.[2] It has been reported that cyromazine can also be converted to melamine in plants.[3][4] NH2 Melamine combines with cyanuric acid to form melamine cyanurate, which has N N been implicated in the Chinese protein export contaminations. http://en.wikipedia.org/wiki/Image:Melamine.svg H2N N NH2 Glycosyl amines (2) Many glycosyl amines occur in Nature and play important roles in living matter. The most important are glycosyl amines derived from D-ribose or 2-deoxy-D-ribose and purine or pyrimidine beses (nucleosides), isolated from the hydrolyzates of nucleic acids. Another important group of glycosyl amines mediates the linkage between sugars and proteins in glycoproteins. Glycosyl amines can be obtained directly from amines with glycoses. Their more advantageous methods of preparation start from glycosyl halogenides or otherwise activated glycoses either directly by treatment with amine or through glycosyl azide followed by its reduction. In synthetic applications, they are being used for preparation of amino saccharides and glycamines (aminodeoxyalditols). Good crystallizing N-(4-nitrophenyl)glycosyl-amines are being used for characterization of sugars. Glycosyl amines of nucleic acids RNA nucleosides: HO N O HO N O OH HO OH HO O N N N HO N NH 2 NH 2 O N NH N HO OH Guanosine O O N N O HO NH 2 Adenosine HO O OH Cytidine NH Uridine DNA nucleosides: HO N O HO N NH 2 N N HO O N HO HO O N O NH N HO NH 2 N HO O O N O HO N NH O NH 2 Deoxyadenosine Deoxyguanosine Deoxycytidine Deoxythymidine D-mannose N-phenyl-β-D-mannopyranosylamine (crystalline compound) The above conversion (and the fact that the analogous N-phenyl-β-D-glucopyranosylamine does not easily crystallize) is being utilized for isolation of Dmannose from the equilibrium mixture of D-glucose and D-mannose (73:27) built up by the Bílik reaction. D- or L-ribose is being isolated similarly from its equilibrium mixture with the respective D- or L-arabinose (~ 1:2) OH OH O MeOH O H OH + OMe OH OH OH D-glucopyranose methyl-α-D-glucopyranoside OH OH OH O O O OH Me OH OH OH OH methyl- -α-D-glucopyranoside OH O OH O OH OH other products + OH OH OH -glycoside (the name includes the anomeric oxygen atom) (≡ glycosyloxy) glycosyl- glycosides (in general) (not O-glycosides !!!) OH O NHPh O OH OH S-ethyl-α-D-thioglucopyranoside (thioglycosides) (not S-glycosides !!!) O OH OH SEt OH OH OH OH OH OH N-phenyl-β-D-glucopyranosylamine (glycosyl amines) (not N-glycosides !!!) OH 2-(β-D-glucopyranosyl)naphtalene (C-glycosyl compounds) (not C-glycosides !!!) • Thioglycosides (not S-glycosides !!!) • Glycosyl amines (not N-glycosides !!!) • C-Glycosyl compounds (not C-glycosides !!!) • Glycosides (not O-glycosides !!!)