MOLES AND GASES
advertisement

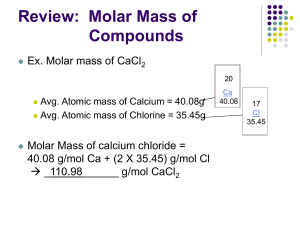
1 mole of any gas at (STP) standard temperature (0C) and pressure (1 atm) has a volume of 22.4 cubic decimeters (dm3), which is the same as 22.4 liters (L). This volume of gas is called a molar volume because it is the volume of 1 mole of a gas at standard conditions. To calculate molar volume of a gas: (X) Moles 22.4 L/mol 1 mol = Molar volume (L) To calculate moles: (÷) Molar volume of gas 1 mol 22.4 L/mol = Moles A room with a volume of 4000 L contains how many moles of air at STP? A chemical reaction produces 0.82 moles of oxygen gas. What volume will that gas occupy at STP? 1.) A container with a volume of 893 L contains how many moles of air at STP? (L mol) 2.) A chemical reaction produces 0.37 mol of N2 gas. What volume will that gas occupy at STP? (mol L) 6. 5.0g of H2 7. 100g of O2