Bioenergetics
advertisement
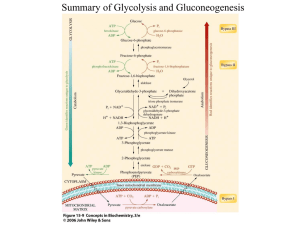
Exercise Physiology: physiology: the sum of all biologic processes what happens to these processes when exercise Metabolism: sum total of all processes occurring in living organisms catabolism: breakdown anabolism: build up Energy transductions or conversions are limited by the laws of thermodynamics Energy is transferred or converted food converted to fat light and CO2 make sugar Measured in kilocalories amount of energy necessary to raise the temperature of 1 lit of water one degree Celsius Energy then, is related to the ability to perform work Can measure the capacity for maximal work capacity in man via exercise physiology 1st law of thermodynamics: energy is neither created nor destroyed it is converted or transformed from one form to another Energy can be categorized as potential energy (amount of energy in canoe on top of falls) – can be light, electric, or bound kinetic energy (amount of energy in canoe at the bottom of falls) – energy of motion Energy-releasing and Energyconserving processes must be coupled exergonic processes release or “frees” energy – downhill endergonic processes release of “store” energy – uphill Transfer of potential energy is unidirectional, to kinetic energy, lowering the energy to do work This parallels the second law of thermodynamics: potential energy gradually decreases, entrophy increases the reactions in the body move towards spontaneity, disorder, and randomness Energy conversions photosynthesis respiration (part of energy converted can be utilized for different work in the human body) Biologic work in humans: Bioenergetics mechanical (muscle contraction) chemical (biosynthesis of cellular molecules) transport (concentrating chemicals in intra and extracellular fluids) Factors affecting the rate of bioenergetics: enzymes coenzymes mass action temperature Hydrolysis and Condensation hydrolysis: complex organic molecules are catabolized to simpler forms for assimilation condensation: molecule of water is formed in this anabolic process Oxidation and Reduction rxns oxidation: transfer of either oxygen or hydrogen atoms, or electrons – loss of electrons, gain of valence – oxidizing agent is electron acceptor reduction: gain of either oxygen or hydrogen atoms, or elctrons – gain of electrons, loss of valence – reducing agent is electron donor together are called redox reaction e.g. NAD to NADH + H or FAD to FADH2 Energy Expenditure Measurements: Direct – bomb calorimetry Indirect – closed circuit – open circuit ATP, energy currency of the body stores small amounts, 85g or 3 oz intermediate compound part of energy receiver-energy donor cycle all energy can be transformed or converted to ATP equivalents ATP equivalent is the energy differential in the conversion of ATP to ADP, and vice versa Cleaving of ATP the cleaving of ATP, hydrolysis of ATP can occur in the presence or absence of oxygen is termed nonaerobic