Chapter 06 Notes
advertisement
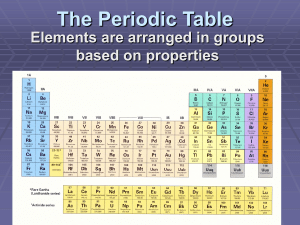
Chapter 6 The Periodic Table The Periodic Table • Dmitri Mendeleev is credited with being the father of the periodic table Mendeleev’s Table • Mendeleev’s Periodic Table was arranged by the elements’ Atomic Mass. • Element 101 in the Periodic Table is named in his honor. Modern Periodic Table • Today’s Periodic Table is arranged by the elements’ Atomic Number. • Elements with similar properties are placed in the same column on the periodic table. Modern Periodic Table • The columns are called groups • The rows are called periods Modern Periodic Table Practice: Find the period and group of the following elements: Period Group • Ca _____ _____ • Al _____ _____ • Zr _____ _____ • Xe _____ _____ • Ra _____ _____ • U _____ _____ Metals, Nonmetals, & Metalloids Metals • Three or fewer electrons in the outer energy level • Good conductors of heat and electricity • Malleable (shapeable) • Metals are hard solids with shiny surfaces Nonmetals • Five or more electrons in the outer energy level • Poor conductors of heat and electricity • Most are gases or brittle solids • Dull surfaces Metalloids • These are elements that have properties of metals and nonmetals. PP: Identify the following elements as metals, nonmetals, metalloids: a) Sulfur (S) b) Hydrogen (H) c) Chromium (Cr) d) Tellurium (Te) 1 2 5 6 9 3 4 7 10 8 Family Names • Alkali metals Family Names • Alkaline earth metals Family Names • Transition metals Family Names • Boron group Family Names • Carbon group Family Names • Nitrogen group Family Names • Chalcogen Family Names • Halogens Family Names • Noble gases Family Names • Lanthanoids Family Names • Actinoids So What’s The Deal With The Shape? • The distribution of electrons around an atom was used to create the shape of the Periodic Table. 6.3 Periodic Trends • The radii or atoms increases from top to bottom and right to left. • PP: Which has the largest radius: – – – – Magnesium (Mg) Silicon (Si) Sulfur (S) Sodium (Na) • The factors that affect the size of an atom are: – Nuclear charge – Number of energy levels Ionic Radii • The radius of positive ions (cation) is smaller than that of the parent atom. + Atom Ion • The radius of negative ions (anion) is larger than that of the parent atom. - Atom Ion PP: Identify the larger particle: a) Na Na+ b) Cl Clc) O O2d) Al Al3+ e) Ca Ca2+ Ionization Energy • Ionization energy is the energy required to remove an electron from an atom. • PP: Which has the largest ionization energy? – – – – Magnesium (Mg) Silicon (Si) Sulfur (S) Sodium (Na) Factors Affecting Ionization Energy A strong nuclear charge will increase ionization energy. Strong shielding effect will decrease ionization energy. A full octet will increase ionization energy. Ionization Energy of sodium, magnesium, and aluminum: Na Mg Al 496 737 577 4563 1450 1,816 6913 7731 2,881 9541 10545 11,600 Explain the discrepancy in ionization energy. Electronegativity • Electronegativity is the ability of an atom to attract the electrons from another atom. • PP: Which has the largest electronegativity: – – – – Magnesium (Mg) Silicon (Si) Sulfur (S) Sodium (Na)