Chapter 6 Review packet
advertisement
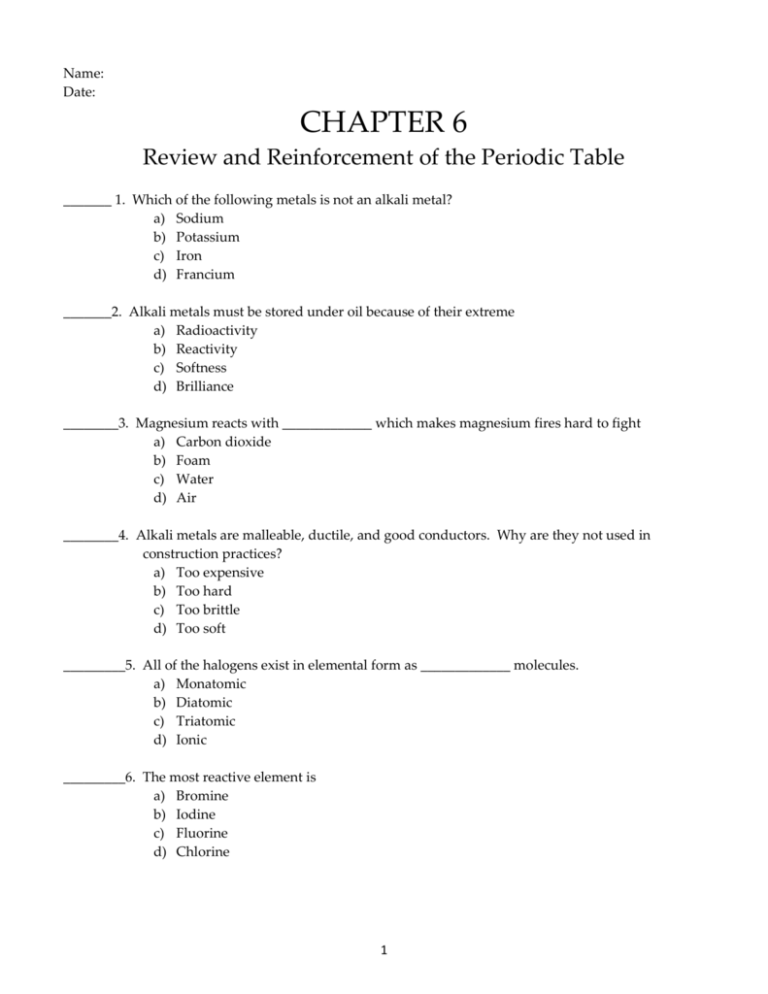
Name: Date: CHAPTER 6 Review and Reinforcement of the Periodic Table _______ 1. Which of the following metals is not an alkali metal? a) Sodium b) Potassium c) Iron d) Francium _______2. Alkali metals must be stored under oil because of their extreme a) Radioactivity b) Reactivity c) Softness d) Brilliance ________3. Magnesium reacts with _____________ which makes magnesium fires hard to fight a) Carbon dioxide b) Foam c) Water d) Air ________4. Alkali metals are malleable, ductile, and good conductors. Why are they not used in construction practices? a) Too expensive b) Too hard c) Too brittle d) Too soft _________5. All of the halogens exist in elemental form as _____________ molecules. a) Monatomic b) Diatomic c) Triatomic d) Ionic _________6. The most reactive element is a) Bromine b) Iodine c) Fluorine d) Chlorine 1 _________7. Natural gas wells are a major source of today’s a) Helium b) Hydrogen c) Argon d) Xenon ________ 8. There are no known compounds of a) Xenon b) Krypton c) Helium, neon, and argon d) Any of the noble gases ________ 9. The formation of nitrogen compounds from nitrogen gas is called a) Nitrogenation b) Purification c) Oxidation d) Nitrogen fixation _________ 10. The majority of a multi-vitamin, by mass, is made up of a) Carbon family compounds b) Nitrogen family compounds c) Transition metal compounds d) Alkaline earth metal compounds 11. Explain how Charles Hall contributed to today’s large-scale production of aluminum. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 12. Why is carbon considered to be “unique” among the elements? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 13. Compare the two forms of elemental oxygen. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 2 14. Why are gold alloys more commonly used for jewelry than pure gold? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 15. Why is iron such a significant metal? What is one of the disadvantages of iron? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 16. What is an alloy? Explain with an example. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 17. Why is beryllium a better choice than iron to use in an alloy for making planes? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 18. What properties distinguish metals from nonmetals? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 3 BUILDING THE PERIODIC TABLE The following elements belong together in families as grouped below. The elements as listed are not necessarily in order. The letters are NOT the normal symbols for the elements. ZRD, SIFP, JXBE, LHT, QKA, WOV, YMC, GUN The assignment is to rearrange these elements in the proper periodic form, according to the information given below. Fill in the answers in the periodic table provided at the bottom of this page. Use the ALCHEM periodic table for assistance if necessary. 1. U has a total of six electrons. (Used as an example below – U is carbon, therefore G and N are either silicon or germanium) 2. A is the second most common element in the atmosphere. 3. E is a noble gas. 4. S is an alkali metal. 5. O is a halogen. 6. O has an atomic number larder than V but smaller than W. 7. The charge on an L ion is 2+. 8. C has 5 electrons in its outer energy level. 9. The atomic mass of T is more than that of H but less than that of L. 10. M has an atomic number one less than A. 11. The electrons of atom N are distributed in 3 energy levels. 12. R has the largest atomic mass of its group. 13. F is a gas at room temperature. 14. Atom B contains 10 protons. 15. Q has an atomic mass less than K. 16. Y is more metallic than either M or C. 17. X has an atomic number one higher than F. 18. D has the smallest mass in its group. 19. P is the most reactive element in its family. 20. J has the greatest density of the elements in its group as listed. 21. Atoms of I are larger than those of S. I II III IV U Dotted lines provide workspace for families GUN 4 V VI VII VIII Review and Reinforcement Reading the Periodic Table On the line at the left, write the number of the appropriate location of each group of elements on the periodic table below. Some letters will be used more than once. ____ ____ ____ ____ ____ ____ ____ 1. Carbon family 2. Alkaline earth metals 3. Inner transition metals 4. Halogens 5. d-block elements 6. Oxygen group 7. Alkali metals ____ ____ ____ ____ ____ ____ ____ 8. f-block elements 9. Noble gases 10. p-block elements 11. Nitrogen family 12. p-block elements 13. Transition metals 14. Group of 1 semi-metal & 4 metals m a k b c d e f g h i j Use your skills to answer the following questions. Below is the abbreviated electron configuration for sodium. Explain each part of this configuration in the space provided. 15. __________________________________________________________________ 16. __________________________________________________________________ [Ne] 3 s 1 17. __________________________________________________________________ 18. __________________________________________________________________ 5 Practice Problems 1. Chlorine, selenium, and bromine are located near each other on the periodic table. Which of these elements is (a) the smallest atom? (b) the atom with the highest ionization energy? 6. Which of the following is the largest: a potassium atom, a potassium ion with a charge of +1, or a rubidium atom? 2. Phosphorous, sulfur, and selenium are located near each other on the periodic table. Which of these elements is the (a) largest atom? (b) the atom with the highest ionization energy? 7. Which of the following is the largest: a chlorine atom, a chlorine ion with a charge of 1-, or a bromine atom? 3. Scandium, yttrium, and lanthanum are located near each other in the periodic table. Which of these elements is (a) the largest atom? (b) the atom with the smallest ionization energy? 8. Which of the following is the smallest: a lithium atom, a lithium ion with a charge of 1+, or a sodium atom? 4. (a) Which of the following atoms is smallest: vanadium, chromium, or tungsten? (b) Which of these atoms has the highest ionization energy? 9. Which of the following is the largest: a tellurium ion with a charge of 2-, an iodine ion with a charge of 1-, or a xenon atom? 5. (a) Which of the following atoms is smallest: nitrogen, phosphorus, or arsenic? (b) Which of these atoms has the smallest ionization energy? 10. Aluminum, silicon, and phosphorous are located near each other in the periodic table. Which of these elements is (a) the largest atom? (b) the atom with the highest ionization energy? 6 APPLY – Two Trendy Elements You can compare the sizes and chemical reactivity of two atoms by looking at their location on the periodic table. The two diagrams at the right show the relative sizes of a sodium atom and a chlorine atom. Use your knowledge of periodic trends to answer the following questions. Sodium atom Chlorine atom 1. Both sodium and chlorine are in the same period on the periodic table. Explain the difference in their sizes. 2. Predict the charge that an ion of each element would have. Explain your answer. 3. Compare the amount of ionization energy required to remove the first electron from each of these atoms. 4. Compare the electron affinities of these atoms. 5. Draw the ion for each atom. Be sure to accurately represent their sizes relative to the original atoms. 6. Explain the ions you have drawn. How do these two elements compare in size now? 7 Review and Reinforcement Periodic Trends Use the periodic table and your knowledge of periodic trends to answer the following questions. Which atom in each pair has the larger atomic radius? _____ 1. Li or K _____ 2. Ca or Na _____ 3. Ga or B _____ 4. O or C _____ 5. Cl or Br _____ 6. Be or Ba _____ 7. Si or S _____ 8. Fe or Au Which ion in each pair has the smaller atomic radius? _____ 9. Na+ or O2- _____ 10. Ba2+ or I- _____ 11. Al3+ or P3- _____ 12. K+ or Cs+ _____ 13. Fe2+ or Fe3+ _____ 14. F- or S2- Which atom or ion in each pair has the larger ionization energy? _____ 15. Na or O _____ 16. Be or Ba _____ 17. Ar or F _____ 18. Cu or Ra _____ 19. I or Ne _____ 20. K or V _____ 21. Ca or Fr _____ 22. W or Se Write the charge that each of the following atoms will acquire when it has a complete set of valence e-. _____ 23. O _____ 24. Na _____ 25. F _____ 26. N _____ 27. Ca _____ 28. Ar 8 Review and Reinforcement: Periodic Trends – CONTINUED 29. Define atomic radius. 30. Why do atoms get smaller as you move across a period? 31. Explain the relationship between the relative size of an ion to its atom and the charge on the ion. 32. Contrast ionization energy and electron affinity in general. In general, what can you say about these values for metals and non-metals? 33. Why is there such a large jump in ionization energy between the second and third ionization energies for magnesium? 34. Explain why noble gases are inert and do not form ions. 35. Define the term electronegativity. What is the periodic trend for electronegativity? 9