Powerpoint for notes
advertisement
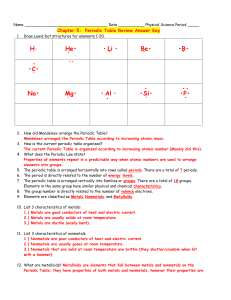
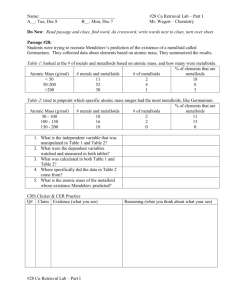
The Periodic Table 6.5A Students will know that an element is a pure substance represented by chemical symbols. The Basics • The periodic table is a chart that is used to organize the elements. • Elements are pure substances that cannot be broken down into smaller substances. • Everything on Earth is made up of these 109 elements. Arrangement of Elements • Elements are listed in order of increasing atomic number. • The atomic numbers get bigger on the chart from left to right going across in rows. • Elements with properties in common are grouped together Symbols • On the periodic table, each element is listed with its element symbol and atomic number; many versions of the table also list the element's atomic mass • Use the key like the one shown below. Why use the Periodic Table? • The main value of the periodic table is the ability to predict the chemical properties of an element based on its location on the table. • Elements can be located quickly and are displayed in an easy to read format Groups • The elements on the periodic table are arranged so that elements with similar properties fall into the same vertical column. • These vertical columns are called groups or families. • Some groups have names such as the Noble gases shown here. Periods • A period is a horizontal row in the periodic table of the elements Metals, Metalloids and Nonmetals • Metals and Nonmetals are separated with a stair-step line • Metalloids touch the line • Metals are shiny, malleable (able to shape), and conductors of heat and electricity • Nonmetals are dull, brittle, and nonconductors or insulators. Many are gases. • Metalloids have characteristics of each. • We will learn more about these groups in another lesson. Metals, Metalloids, & Nonmetals Notice that Aluminum (Al) touches the line but is a metal. Hydrogen (H) is on the other side and is a nonmetal. On Your Copy of the Periodic Table… • Draw arrows for : • Periods • Groups Metals, Metalloids, and Nonmetals • You know that Oxygen is a gas and IS NOT a metal. Find it and circle it. Above this section, write NONMETALS. Nonmetals Metalloids and Exceptions • Now, trace the stair-step line and write in metalloids. • Remember Aluminum and Hydrogen are exceptions. Mark them with a light X. metalloids Metals • Find Gold (Au). • You know that it is a metal. • Circle it and write METALS in the section above it. Metals metalloids • Now, you have all the information you need ready on your periodic table.