Plasticizers - sciensage.info
advertisement

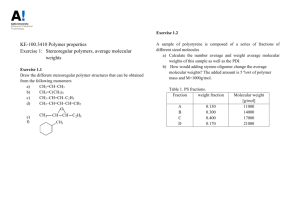
Polymer Structure & Synthesis Determination of Molecular Weight and Molecular Weight Distribution Cryoscopy Freezing point of a solvent is lowered when a nonvolatile solute is dissolved in it. Freezing point of the solution will be depressed. This phenomenon of depressing the freezing point of a liquid by the addition of a solute is known as Cryoscopy. The extent of the freezing point depends on the number of solute molecules dissolved per unit volume of solution and its independent of the size and nature of the solute molecule. Polymer & Process Engineering Polymer Structure & Synthesis Continue….. So, Number average molecular wt. is measured by this technique. If depression of the freezing point is denoted by Tf , Its related to the Number-average Molecular wt. and concentration through following equation. Tf /C = (RTf / Hf ) 1/M+BC 2 Where, C is concentration of the solution, ρ the density of the solvent, R the universal gas constant; Tf the freezing point of the solvent, B is a constant and ΔHf is the heat of fusion of the solvent. Polymer & Process Engineering Polymer Structure & Synthesis Continue….. Graphical representation. To find out the freezing point, solvent or solution is carefully supercooled to a temperature that is about 5C lower than the freezing point. Once the freezing points of the solvent and solutions Tf different concentrations are known, ΔTf can be calculated for all these concentrations. Drawback of this method is, measurement of temperatures. Highly precised temperature sensors are required. This method can measure molecular wt. upto 30,000. Polymer & Process Engineering Polymer Structure & Synthesis Ebulliometry This technique is based on boiling point of solutions. Boiling point of solutions is higher than the pure solvents. This phenomenon is used to determine the molecular wt. of polymers. Tb /C=(RTb / Hv ) 1/M n +BC 2 Where ΔHv and Tb are the heat of vaporization and boiling of the solvent. Polymer & Process Engineering Polymer Structure & Synthesis Continue….. There are two types of ebulliometers. In first type, the solution of the polymer whose molecular wt. is to be determined is heated in a boiler. The solution is then brought in contact with a sensor. Another in pure solvent. In second type, both of solution and pure solvent are heated in identical containers and uniform boiling is achieved. It can also be used to determine the molecular wt. upto 30,000. Polymer & Process Engineering Polymer Structure & Synthesis Membrane Osmometry It is most widely used technique to determine number average molecular wt. It is based on the phenomenon of osmosis. What is Osmotic pressure? /RTC=1/M n +BC Π is the osmotic pressure. Polymer & Process Engineering Polymer Structure & Synthesis Vapor Phase Osmometry This technique is based on the principle that at a given temperature the vapor pressure of a solution is less than that of the pure solvent. The relationship between the vapor pressure difference of solvent and solution, P=(p -p ) and number average molecular wt. is given as 0 1 limc 0 P/C o 1 o =-p 1 V1 /M Experimental method. Polymer & Process Engineering Polymer Structure & Synthesis