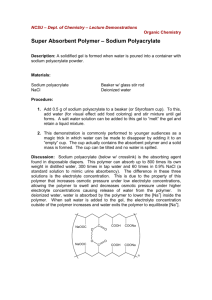
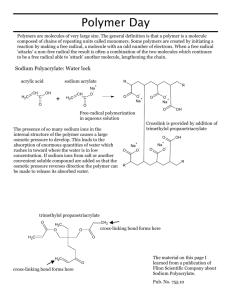
Sodium polyacrylate
advertisement

Superabsorbent Polymer, Sodium polyacrylate • It is a polymer where the long chains are cross-linked to one another. • It is a polymer that can absorb water many times its volume therefore earning the term superabsorbent. • After it absorbs the water it forms a stiff gel. Why sodium polyacrylate absorbs water • It is a long chained molecule with many repeating units (a polymer). • Each of the repeating units has a portion that holds an electrical charge. • The electric charges on the polymer attract water molecules and bind them to the polymer. • Each charge binds several water molecules and each molecule of polymer can bind a large volume of water. Procedure • Remove cotton-like filling from diaper • tear filling into small pieces and put in the plastic bag • rub bag in hands to separate the polyacrylate from the cotton • put the polyacrylate in a dry beaker removing any visible cotton • slowly add liquid into beaker stopping to make sure it is absorbed, continue adding liquid until no more is being absorbed • measure the volume of liquid absorbed Questions • Why does something dissolved in the water reduce the amount of water absorbed by the polymer? • What are some uses of this material? Why the salt decreases the amount of water absorbed • Like the polymer the salt also contains electrical charges which attract water molecules. • Furthermore, the charges in the salt are attracted to the charged parts of the polymer and displace the molecules of water from the polymer. Other uses of sodium polyacrylate • • • • • • filtering fuels food packaging potting soil alkaline batteries hair gels fire retardants