TALK PPTX
advertisement

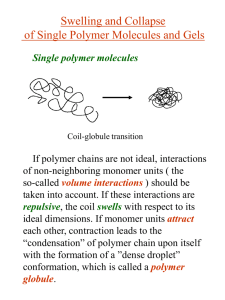
APS March Meeting 2012 Padden Award Symposium February 28, 2012 Influence of Charge and Network Inhomogeneities on the Swollen-Collapsed Transition in Polyelectrolyte Nanogels Prateek Jha (Northwestern University) Jos Zwanikken (Northwestern University) François Detcheverry (University de Lyon) Juan de Pablo (University of Wisconsin-Madison) Monica Olvera de la Cruz (Northwestern University) This material is based upon work supported by NSF under Award numbers DMR-0907781 and DMR-0520513. Polymer Nanogels • Finite-sized, solvent-permeated polymer networks • Highly responsive materials – High volume changes by absorbing/releasing solvent – Small size (10-1000 nm) enables rapid kinetic response – Ionic (Polyelectrolyte) nanogels superior than neutral nanogels Kabanov and Vinogradov, Angew Chem Int Ed Engl. 2009 ; 48(30): 5418–5429 Polyelectrolyte Nanogels as Drug Delivery Carriers and in Anti-Cancer Therapy • Can easily incorporate oppositely charged drugs/biomacromolecules e.g. oligonucleotides, siRNA, DNA, proteins, … Kabanov and Vinogradov, Angew Chem Int Ed Engl. 2009 ; 48(30): 5418–5429 • High swelling of nanogel used in killing cancer cells Park et al., Journal of Controlled Release 135 (2009) 89–95 Classical Description of Gel Swelling Rubber Elasticity Flory-Huggins Theory Donnan Membrane Equilibrium • Homogeneous deformation uniform volume fraction, Nv / V • Charge neutrality in gel and solvent bath No Coulomb interactions • Electrostatic contribution from mobile ion entropy (Donnan) – Mobile ion concentrations from association dissociation equilibrium of salt • Free Energy of gel: F Felastic FFH FDonnan • Condition of Mechanical Equilibrium: Osmotic Pressure : F / V 0 Solve for Flory, Principles of Polymer Chemistry Charge and Network Inhomogeneities in Nanogels 8lB cs N Av 1 1 / 2 e2 where lB 4k BT 1 10 100 nm for cs 0.001 0.00001M for wat erat room temperature Influence on Swelling behavior ? Polymer Volume Fraction • Nanogels are not homogeneous – Crosslinking inhomogeneities (during preparation) – Excess Charge due to presence of surfaces • Excess Charge Coulomb Interactions (Neglected in the Donnan picture) • Important when nanogel size is comparable to screening length Distance from center [nm] J Ramos et al; Soft Matter, 2011, 7, 5067-5082 Poisson-Boltzmann Description Network charge fixed and homogeneous f Charge fractionof polymer F r , c r , c r c r ln c r c r ln c r dr k BT 1 r r dr 2 PoissonEquation: 2 r 4lB r • Mobile ion concentrations by mean-field approximation F / c 0 c cs exp • Dielectric mismatch between solvent and polymer can be incorporated by a solvation term: c cs exp ESolvation cs exp Gel Solvent Bath PK Jha et al, Current Opinion in Solid State and Materials Science, 15(6), 271-276 (2011) • Smaller, collapsed, and gels at low salt concentration or in high dielectric solvent have larger excess charge Donnan theory fails PK Jha et al, Current Opinion in Solid State and Materials Science, 15(6), 271-276 (2011) • Excess charge may be used to self-assemble nanogels to crystalline structures Gottwald et al, Phys. Rev. Lett. 92, 068301 (2004) • Dielectric mismatch can give rise to re-entrant behavior: re-swelling at high salt concentration Salt Concentration Theoretically Informed Coarse-Grained Simulations (TICG) Detailed swelling behavior Crosslink Inhomogeneities Fluctuations Few physical invariants Free of discretization effects Computationally Efficient • Construct the free energy functional: H ri ,(r), c (r) Ubonded ri Ηnon-bonded (r), c (r) • Bonded (Elastic) Contribution from Gaussian Chain Approximation • Non-Bonded Contribution = Solvent + Entropy + Coulomb • Monte Carlo using the free energy functional Detcheverry et al, Macromolecules, 2008, 41 (13), pp 4989–5001; Soft Matter, 2009, 5, 4858-4865; Faraday Discuss., 2010, 144, 111-125 Effect of Polymer Charge GOOD SOLVENT POOR SOLVENT • Very high swelling for ionic nanogels (Coulomb repulsions) • Discontinuous volume transition for ionic nanogels PK Jha et al, Soft Matter, 7, 5965-5975 (2011) Effect of Salt Concentration Swelling decreases with increase in salt concentration (screening) PK Jha et al, Current Opinion in Solid State and Materials Science, 15(6), 271-276 (2011) Summary • Density and Charge Inhomogeneities strongly contributes to the nanoscale gel behavior • New Simulation Scheme for swelling behavior of polyelectrolyte nanogels • Discontinuous volume transition for ionic nanogels SWOLLEN COLLAPSED Good Solvent Bad Solvent Highly Charged Neutral/Weakly Charged Low salt High salt Loosely crosslinked Tightly Crosslinked