Zinc - elementssph-7-4

Z INC -Z N
Brenda 7.4
G
ENERAL
I
NFO
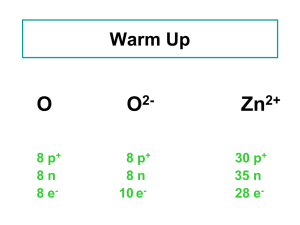
Atomic number: 30
Melting Point: 419.58 C
Boiling Point: 907 C
Element Classification:
Metal
D
ISCOVERY
First produced in the
1400s in India
Was rediscovered by
Andreas Sigismund
Marggraf in 1764
D
ESCRIPTION
It is a soft metal
It a has grayish or blue-white color
It contains 21 isotopes where 5 of them is stable and the other 16 are not (unstable)
It is brittle at low temperatures but malleable at
100-150 C
It is an electric conductor
It burns in air at high red heat
T HIS IS WHAT Z INC LOOKS LIKE …
USES OF
Z
INC
1. F ORM NUMEROUS ALLOYS
Such as…
Brass
Bronze
Nickel Silver
Soft Solder
German Silver
Spring Brass
Aluminum Solder
2. M
AKE DIE
CASTINGS
In..
Electrical
Automotive
Hardware
3. T O GALVANIZE OTHER
METALS
To prevent corrosion
Galvanize means to put coating on iron
To make metals stop rusting for quite a long time
I NTERESTING F ACTS
Zinc is..
Found on roofs because it has a very high quality of roofing material
A metal we all need for life
Part of enzymes and many biological medicated processes
Easily reacted with acids to produce hydrogen
Very slow to oxidize
An interesting element because it is not a poisonous element, where the two other elements that are in the same group with Zinc, Mercury and Cadmium, are both pretty poisonous.
Something we need for us to survive
H AZARDOUS E FFECTS OF
Z INC TO THE E NVIRONMENT
Zinc production can cause:
Water pollution-it can cause for fish to be accumulated with Zinc which is able to bio magnify up the food chain
Soil pollution-farmlands could be polluted which can cause farm animals to absorb concentration that damages their health. It could also break down microorganisms and earthworms since their habitats are polluted.
Plants to have their system unstable, unhandled and uncontrolled
M ORE INFORMATION ABOUT
Z INC
In our body, if we don’t have enough Zinc, we are not able to smell things.
When you combine it with other elements, it could get quite frisky
Zinc is an abundant metal
An enzyme called ‘Carbonic Anhydrase’ needs Zinc to catalyze the reaction of CO2 and water
B IBLIOGRAPHY
Websites
Helmenstine, Anne Marie. “Zinc Facts.” About.com. 25 April 2010 http://chemistry.about.com/od/elementfacts/a/zinc.htm
“Zinc-Zn.” Lenntech. 25 April 2010 http://www.lenntech.com/periodic/elements/zn.htm
“The Element Zinc.” Jefferson Lab. 25 April 2010 http://education.jlab.org/itselemental/ele030.html
Images
“Andreas Sigismund Marggraf.” Photo. 25 April 2010 http://www.bbaw.de/akademie/kalender/biog-pic-009-marggraf.jpg
“Zinc.” Photo. 25 April 2010 http://z.about.com/d/chemistry/1/0/w/c/zinc.jpg
“Zinc Crystal.” Photo. 25 April 2010 http://www.albany.edu/offcourse/july98/zinc_sm.jpg
“Zinc.” Photo. 25 April 2010 http://www.webelements.com/_media/elements/element-picstheo/30_Zn_1.jpg
“Zinc Rangehood.” Photo. 25 April 2010 http://christopherleeplummer.com/company/blog/wpcontent/uploads/2007/09/zinc-rangehood.jpg
“5" Seat Springs (Brass).” Photo. 25 April 2010 http://www.justifieddefiance.com/catalog/images/Brass4Springs.jp
g
“Zinc Die Casting-01.” Photo. 25 April 2010 http://www.bldiecasting.com/products_img/200661692428.jpg
“Results for rust.” Photo. 25 April 2010 http://bostonbiker.org/files/2009/12/rust3.jpg
“Roof tiles texture.” Photo. 25 April 2010 http://mayang.com/textures/Architectural/images/Roofing/roof_tile s_2260555.JPG
“Degradation of earth’s land surfaces-land pollution.” Photo. 25
April 2010 http://www.gradebook.org/pollution4.gif
“Carbonic Anhydrase.” Photo. 25 April 2010 http://andromeda.rutgers.edu/~huskey/images/carbonic_anhydras e_active_site_w.png
Website
(2010, 25 April) Zinc - Periodic Table of Videos Retrieved from http://www.youtube.com/watch?v=99wPiMb-k0o