Voltaic Cells - EARJ Chemistry
advertisement

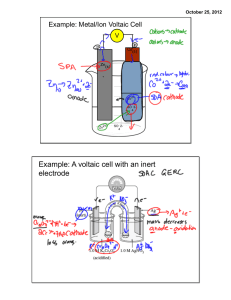
http://www.wwnorton.com/college/chemistr y/gilbert2/tutorials/interface.asp?chapter=c hapter_18&folder=zinc_copper_cell Voltaic Cells or Batteries • Rechargeable batteries power a great deal of the electronics we use today, including cordless phones, cell phones and laptop computers. Voltaic cells, as well as various other electrolytic cells, are used in these rechargeable batteries • Make your own iPod/iPhone/GPS/etc… battery-pack and recharger! The charger circuitry and 2 AA batteries fit into an small space such as an Altoids gum or mint tin, and will run your iPod for hours, 2.5x more than you’d get from a 9V USB charger! You can use rechargeable batteries too. Recycling cell phones http://atitudesustentavel.uol.com.br/blog/2012/09/25/empresa-recolhe-celulares-para-reciclagem/ Voltaic Cells • Invented by Alessandro Volta in 1800. • Voltaic cells: electrochemical cells used to convert chemical energy into electrical energy • A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The important parts of a voltaic cell: • The anode is an electrode where oxidation occurs. • The cathode is an electrode where reduction occurs. • A salt bridge is a chamber of electrolytes necessary to complete the circuit in a voltaic cell. • The oxidation and reduction reactions are separated into compartments called half-cells. • The external circuit is used to conduct the flow of electrons between the electrodes of the voltaic cell. • A light bulb is a example of a simple load where current (a flow of electrons) is used to resistively heat a filament of metal, usually tungsten, until it radiates energy in the form of visible light. Zn and Cu Cell How does it work????? http://www.wwnorton.com/college/chemistry/gilbert2/tutorials/interface.asp?chapter= chapter_18&folder=zinc_copper_cell • If we are using two metals such as Zn and Cu, we must decide which one will undergo oxidation and which will undergo reduction by looking at the reduction table: Zn+2 + 2e => Zn Cu+2 + 2e => Cu -0.76v +0.34v Cu2+ has a greater tendency to be reduced. Zn will be oxidized. Steps of a Voltaic Cell • e- created at anode – Shown in oxidation half-reaction • e- leave zinc and pass through wire • e- enter cathode and cause reduction – Shown in halfreaction • Positive and negative ions pass through salt bridge to finish the circut Figure 21.5 A voltaic cell based on the zinc-copper reaction. Oxidation half-reaction Zn(s) Zn2+(aq) + 2e- Reduction half-reaction Cu2+(aq) + 2eCu(s) Overall (cell) reaction Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Voltaic Cell Shorthand • Oxidation half cell is listed first with reduced and oxidized species separated by a line. • Reduction is next in the opposite order. • Double line separates the two and represents a salt bridge. Zn|Zn2+||Cu2+|Cu Voltaic Cell Shorthand • Draw shorthand notation for a Mg-Pb cell where the nitrate ion is present. You might want to refer to the activity series to determine what is oxidized and what is reduced! – Draw a diagram for this voltaic cell • Label: anode, cathode, direction of eflow • Write-out the ½ reactions and combined reaction. • If we are using two metals such as Mg and Fe, we must decide which one will undergo oxidation and which will undergo reduction by looking at the reduction table: Mg+2 + 2e => Mg Fe+2 + 2e => Fe -2.37v -0.45v