ch3b_SP13x
advertisement
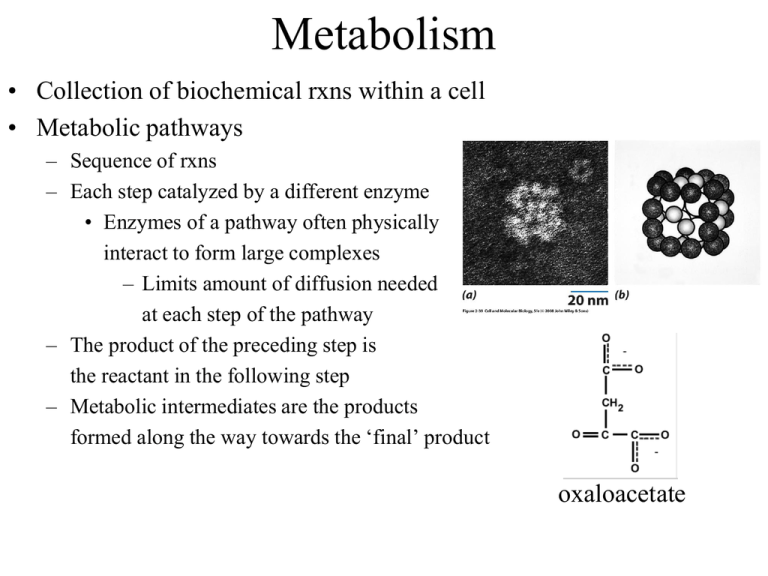
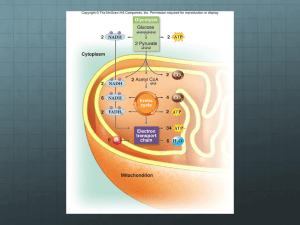
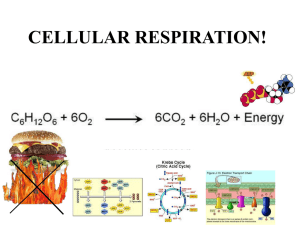
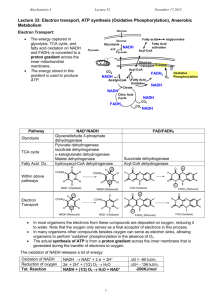
Metabolism • Collection of biochemical rxns within a cell • Metabolic pathways – Sequence of rxns – Each step catalyzed by a different enzyme • Enzymes of a pathway often physically interact to form large complexes – Limits amount of diffusion needed at each step of the pathway – The product of the preceding step is the reactant in the following step – Metabolic intermediates are the products formed along the way towards the ‘final’ product oxaloacetate Catabolism vs Anabolism • Catabolic: breakdown from complex to simple – Yield raw materials (amino acids, etc) and chemical energy (NADH, ATP) – Convergent: diverse starting materials broken down to conserved set of intermediates (pyruvate, Acetyl-CoA) • Anabolic: synthesis from simple to complex – Consume raw materials and chemical energy stored in NADPH and ATP – Divergent: small set of molecules assembled into a diversity of products Anabolism Catabolism Catabolism vs Anabolism Oxidation and reduction • Redox reactions: the gain (reduction) or loss (oxidation) of electrons – Reducing agents = lose e- = get oxidized – Oxidizing agents = gain e- = get reduced Fe0 + Cu2+ <---> Fe2+ + Cu0 Reducing agent + oxidizing agent <---> oxidized + reduced – Metals show complete transfer of e• Reducing agents reduce the charge on oxidizing agents Oxidation and reduction • Redox reactions: the gain (reduction) or loss (oxidation) of electrons – Changes in organic molecules shift the degree of e- sharing • Carbon in C-H bond is reduced • Carbon in C=O bond is oxidized – EN diffs result in e- spending less time around C when bonded to O CH4 + 2O2 --> CO2 + 2H2O Capture and Use of E • Alkanes are highly reduced organic compounds (E rich) – Not well tolerated by most cells • Fatty acids and sugars are well tolerated C6H12O6 + 6O2 --> 6CO2 + 6H2O ADP + Pi --> ATP • Theoretical Yield ~ 93 ATP • Actual (aerobic) ~ 36 ATP ΔG°’= -686 kcal/mol ΔG°’= +7.3 kcal/mol 39% efficient – Marathon runner • Actual (anaerobic) = 2 ATP – Sprinter 2% efficient Glycolysis • Glucose + 2NAD + 2ADP + 2Pi --> 2pyruvate + 2ATP + 2NADH ΔG for actual cell conditions K’eq ΔG°’ • Kinase: an enzyme that can transfer phosphate from ATP to another molecule • Phosphatase: hydrolyzes phosphate from a molecule • Isomerase: an enzyme that can catalyze structural rearrangements • Steps 1-3: 2 ATP used • Aldolase: an enzyme that cleaves an aldol (which is a beta-hydroxy ketone) Two modes of E extraction • 1. Extraction of H+ and 2e- (:H-) – NAD+ + H: --> NADH – Extraction of :H- is done by dehydrogenase enzymes • Dehydrogenase: oxidizes substrates by transferring hydride (H-) ions to an electron acceptor (e.g. NAD+). Nicotinamide Adenine Dinucleotide (NAD) • add :H- to the nicotinamide ring • Most NADH destined for electron-transport chain • Add phosphate to ribose 2’-OH creates NADP/NADPH rAMP • Another example of an ES complex with a covalent intermediate • Regenerate enzyme in last step using inorganic phosphate (Pi) Two modes of E extraction • 2. Substrate level phosphorylation of ADP --> ATP – transfer of phosphate from higher energy compounds to lower energy ones • ATP is not the highest energy compound • Reverse reaction looks like a classic kinase • Mutase: shifts the position of a functional group • aka as a hydratase Glycolysis: summary • Steps 1, 3 – 2 ATP consumed • Step 4 – 6C sugar split into two 3C sugars • Step 6 – Redox reaction: NAD+ + :H- --> NADH • Step 7, 10 – Substrate level phosphorylation • Glucose + 2NAD+ + 2ADP + 2Pi --> 2Pyruvate + 2ATP + 2NADH • No O2 used, anaerobic Reducing power: NADH vs NADPH • Synthesis of fats from sugar requires reduction of metabolites H-C-OH + :H- + H+ ---> H-C-H + H2O • NADH is generated from Catabolic pathways NADH + NADP+ <---> NAD+ + NADPH transhydrogenase • NADPH is used as reducing agent for Anabolic pathways Fermentation can regenerate NAD+ • Under anaerobic conditions – Skeletal muscle: Pyruvate + NADH ---> Lactate + NAD+ – Yeast: Pyruvate ---> Acetaldehyde + CO2 Acetaldehyde + NADH ---> Ethanol + NAD+ - O2 Fermentation can regenerate NAD+ • Under anaerobic conditions – Skeletal muscle: Pyruvate + NADH ---> Lactate + NAD+ – Yeast: Pyruvate ---> Acetaldehyde + CO2 Acetaldehyde + NADH ---> Ethanol + NAD+ • Under aerobic conditions – Pyruvate enters TCA cycle – NAD+ regenerated by electron transport chain (oxidative phosphorylation) + O2 Regulation of enzyme activity • Allosteric modulation (Allostery) – Binding of a molecule to the enzyme activates or inhibits it – Binding occurs at an ‘allosteric site’ on the enzyme – Feedback inhibition: • Final product of a pathway inhibits the first enzyme in the pathway • Keeps level of product from getting higher than needed • A + B --> C + D --> E • E is an allosteric inhibitor that binds to allosteric site blocking 1st rxn Allosteric regulation of metabolism • Most cells have enzymes for both glycolysis and gluconeogenesis • Allostery controls which pathway is active versus inhibited to provide sensitivity to energy needs Allosteric regulation of metabolism ATP --> ADP + Pi ADP + ADP --> ATP + AMP ATP = allosteric inhibitor AMP = allosteric activator AMP = allosteric inhibitor Regulation of enzyme activity by covalent modification • Phosphorylation – Serine uncharged charged H2C-OH --> H2C-O-PO32protein kinases enz P enz protein phosphatases – Threonine also subject to phosphorylation – Tyrosine also subject to phosphorylation – These subtle changes to the chemical information guiding protein folding can yield conformational changes in protein structure that increase or decrease enzyme activity Metabolism: cell overview