lec33_F2015
advertisement
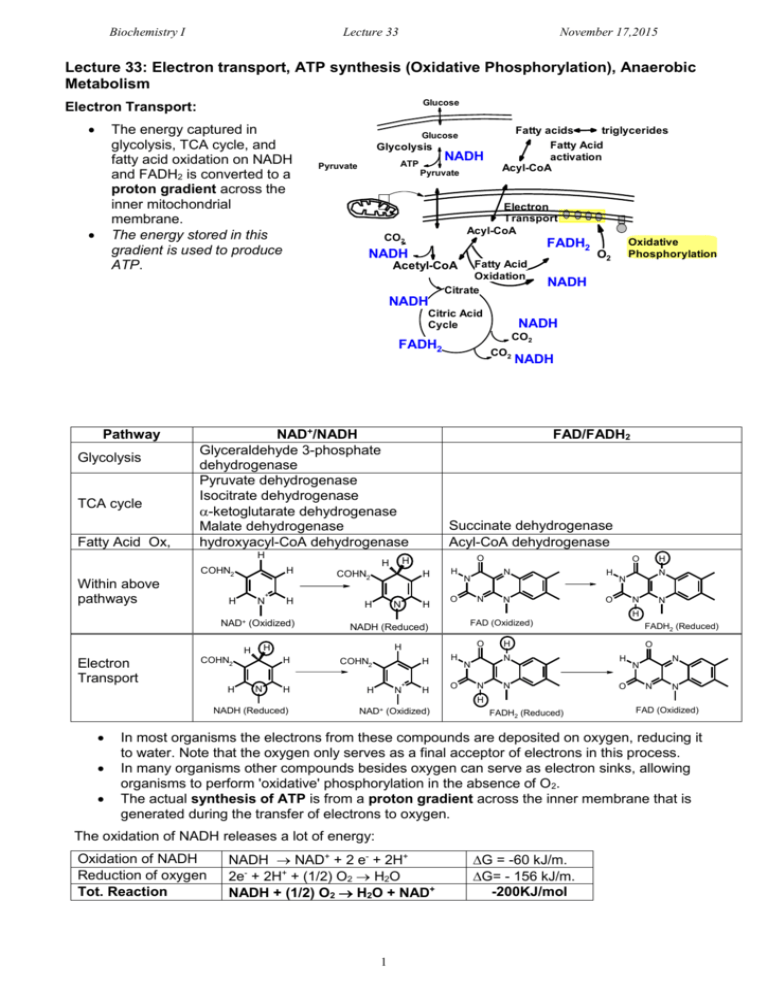
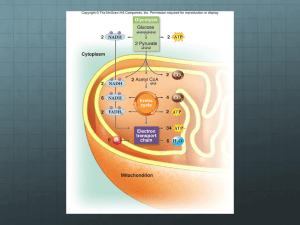
Biochemistry I Lecture 33 November 17,2015 Lecture 33: Electron transport, ATP synthesis (Oxidative Phosphorylation), Anaerobic Metabolism Glucose Electron Transport: The energy captured in glycolysis, TCA cycle, and fatty acid oxidation on NADH and FADH2 is converted to a proton gradient across the inner mitochondrial membrane. The energy stored in this gradient is used to produce ATP. Glucose Glycolysis ATP Pyruvate NADH Pyruvate Fatty acids triglycerides Fatty Acid activation Acyl-CoA Electron Transport Acyl-CoA CO2 FADH2 NADH Fatty Acid Oxidation Citrate Acetyl-CoA Oxidative Phosphorylation O2 NADH NADH Citric Acid Cycle FADH2 Pathway Glycolysis TCA cycle Fatty Acid Ox, NAD+/NADH Glyceraldehyde 3-phosphate dehydrogenase Pyruvate dehydrogenase Isocitrate dehydrogenase -ketoglutarate dehydrogenase Malate dehydrogenase hydroxyacyl-CoA dehydrogenase H COHN2 Within above pathways H + H N H N NADH FAD/FADH2 Succinate dehydrogenase Acyl-CoA dehydrogenase O H COHN2 H CO2 H H NADH CO2 H H N O N O N H N O H N N N N H NAD+ (Oxidized) Electron Transport H N O H H H COHN2 FAD (Oxidized) NADH (Reduced) H COHN2 H H H + N H H O N N FADH2 (Reduced) H O N H N O N N N N H NADH (Reduced) NAD+ (Oxidized) FADH2 (Reduced) FAD (Oxidized) In most organisms the electrons from these compounds are deposited on oxygen, reducing it to water. Note that the oxygen only serves as a final acceptor of electrons in this process. In many organisms other compounds besides oxygen can serve as electron sinks, allowing organisms to perform 'oxidative' phosphorylation in the absence of O2. The actual synthesis of ATP is from a proton gradient across the inner membrane that is generated during the transfer of electrons to oxygen. The oxidation of NADH releases a lot of energy: Oxidation of NADH Reduction of oxygen Tot. Reaction NADH NAD+ + 2 e- + 2H+ 2e- + 2H+ + (1/2) O2 H2O NADH + (1/2) O2 H2O + NAD+ 1 G = -60 kJ/m. G= - 156 kJ/m. -200KJ/mol Biochemistry I Lecture 33 November 17,2015 Key Components in Electron Transfer: 1. Inorganic carriers of electrons b) Fe in heme – e.g. cytochrome C a) Iron-sulfur centers (e.g. succinate dehydrogenase) Methionine S S Cys S Fe Cys S S Fe2+ S Cys Fe S H3C N Fe2+N N N H3C H3C Cys R1 Fe3+ N Fe2+ R1 Fe3+ CH3 N H 2. Organic Carriers of electrons: a) Coenzyme Q. Coenzyme Q is a non-polar electron carrier that diffuses freely in the fluid mitochondrial membrane. R group is non-polar. Can participate in one or two electron redox transactions, two electron reduction shown on the right O OH OMe CH3 OMe CH3 OMe R OMe R O Coenzyme Q OH ubiquinol (hydroquinone) Oxidized Reduced Electron Transport: Gibbs Energy & Flow Chemical Potential NADH Complex I Succinate Complex IIA [FADH2] Q Fatty acid ox. Complex IIB [FADH2] Complex III Complex IIA = succinate dehydrogenase (TCA cycle) Complex IIB = acyl-CoA dehydrogenase (fatty acid oxidation) Cytochrome C Glucose Acyl-CoA Cytosol Pyr Outer membrane Inner membrane Cytochrome C III I IIB IV IIA FADH2 FAD FADH 2 Pyr AcylCoA Succinate NADH acetyl-CoA TCA Fumarate Succinate Dehydrogenase NADH FADH2 FAD NADH 2 Mito Matrix Complex IV Biochemistry I Lecture 33 November 17,2015 Complex I: NADH-CoQ oxidoreductase Four protons/NADH are pumped from the inside (matrix) to the intermembrane space. Complex II: Succinate-CoQ oxidoreductase Succinate dehydrogenase of the citric acid cycle is part of this complex. Two electrons from FADH2 are transferred to CoQ via Fe-S clusters, generating CoQH2. Does not pump any protons. Complex III: CoQH2-cytochrome c oxidoreductase Transfers electrons from CoQH2 to cytochrome c one electron at a time. Four protons are pumped/NADH or FADH2 Cytochrome C: Shuttles one electron from III to IV. Complex IV: Cytochrome c oxidase Accepts 4 e- , one at at a time from cytochrome c. Donates a total of four electrons/O2. Site of oxygen reduction to water. i) Produces 2 water molecules/O2 molecule. ii) Pumps an additional two protons/NADH or FADH2. Energy Stored in the Proton Gradient The energy 'stored' in a concentration gradient can be considered to consist of two parts: GTOTAL GCONC GELEC Defining the reaction direction from inter-membrane space (out) to the matrix (in): i) The Gibbs energy due to a concentration difference across a sealed membrane. The Gibbs energy is: G G 0 RT ln 4 H+/2e I 2 H+/2e 4 H+/2e II QH2 III Q NADH NAD+ IV O2 H2O [ X IN ] [ X IN ] 0 0 ( IN OUT ) RT ln [ X out ] [ X out ] Since the standard chemical potential (0) for the species ([X]) is the same on both the inside and the outside of the membrane: GCONC RT ln [ X ] IN [ X ]OUT This is the amount of energy that is released when the concentration gradient moves towards equilibrium. ii) Movement of a charged particle through a voltage difference. The free energy associated with moving a particle of charge Z, through a voltage difference (=ΔV), is: GELEC = ZF Z = the charge on the transported ion (+1 in the case of the proton) F is Faraday's constant, 96,494 C/mol. C=coulomb is the voltage difference across the membrane, in volts. This voltage difference is often referred to as the membrane potential: = VIN – [ H ] IN GTOTAL RT ln ZF VOUT. [ H ]OUT The total Gibbs free energy is the sum of these two terms: Example Calculation: Typical values across the inner mitochondrial membrane are: [H+]IN/[H+]out = 0.1 (pH=6.5 outside, 7+ on the outside of the membrane: G (8.31)(300 ) ln( 0.1) (1)(96,000 )(0.150 ) 5.7 k J / mol 14.4k J / mol 20 k J / mol 3 Biochemistry I Lecture 33 November 17,2015 ATP Synthesis: ATP synthesis is attained by coupling the free energy of a proton gradient to the chemical synthesis of ATP. The enzyme that accomplishes this coupling is called ATPsynthase (also known as FoF1 ATPase) 3 H+ = 1 ATP synthesized Structural Features: 1. The Fo Complex Membrane-spanning, multi-protein complex. Responsible for coupling the movement of three protons to 120° rotations of the γsubunit. 2. The F1 Complex Attached to Fo, it protrudes into the mitochondrial matrix. Composed of five different subunits: 33 The subunit is the shaft at the center of the 3 3 disk. rotates 120o every time 3 protons pass through the complex. The subunits are asymmetric due to their interactions with the -subunit. 1. One conformation of the subunit has very low affinity for both ADP and ATP. Everything is released. 2. One conformation of the subunit has high affinity for ADP and Pi. 3. One conformation of the subunit makes ATP lower in energy than ADP+Pi. High affinity ADP+Pi High affinity ATP Low Affinity How the motor works: Every time three proton move through the complex, the subunit rotates 120°. The rotation of subunit changes the conformation of the β-subunits such that the Gibbs energy of the bound ADP + Pi becomes higher than the energy of ATP, thus ATP forms spontaneously from the bound ADP and Pi. The newly-formed ATP is released with the transport of three additional protons. The actual synthesis, or formation of the bond between ADP and PI, is catalyzed by conformational changes of the -subunit that occur as a consequence of the rotation. Since all three β subunits are functioning at the same time, the transport of 9 protons in a complete cycle produces 3 ATP. High affinity for ADP+Pi HIgh affinity for ATP (ATP lower in energy than ADP & Pi) Low Affinity NADH FADH2 ~10 protons pumped ~6 protons pumped ~ 3 ATP ~2 ATP 4 Biochemistry I Lecture 33 November 17,2015 Anaerobic Metabolism and the Fate of Pyruvate. Anaerobic Metabolism: NAD+ is required as the electron acceptor in glycolysis. Which step in glycolysis does this occur? Glucose NAD+ People NADH How is NAD+ regenerated? Pyr Lactate Ala [pyruvate dehydrogenase] What happens to glycolysis if NAD+ cannot be regenerated? Yeast Fatty Acids Acetyl CoA NADH [citrate synthase] TCA Cycle NAD+ ethanol Other Fates of Pyruvate i) Pyr can be converted to Acetyl CoA, a one way reaction in humans. a) acetyl CoA can be oxidized by the TCA cycle. b) acetyl CoA can be used to synthesize fatty acids (via citrate), which are then used to make triglycerides. ii) Pyruvate can be converted to alanine in a one-step transaminase reaction. iii) Pyruvate can be used to make oxaloacetate, to replace the carbons that are removed from the citric acid cycle by anabolic processes (this reaction is the first step in gluconeogenesis). Cooperation between muscle and liver cells during active exercise (Cori cycle). a) During intense exercise muscle tissue cannot get sufficient oxygen to process the NADH produced in metabolism. b) Pyruvate is reduced to lactate, to regenerate NAD+ for glycolysis. c) The lactate travels to the liver, where it is oxidized to pyruvate, used to make more glucose, which is then returned to the muscle. Muscle Cell Liver Cell Glucose Hormone Receptor Glucose Transporter glycogen synthase Glycogen Glucose Lactate Lactate Transporter Glycogen F6P Glycolysis F6P Glycolysis PFK Lactate F16P NAD+ NADH Lactate Transporter Glucose glycogen phosphorylase (Glu - Pyr) Lactate Glucose Transporter glycogen synthase Glucose glycogen phosphorylase (Glu - Pyr) Hormone Receptor PFK Gluconeogenesis (Pyr-Glucose) bisPhosphatase F16P NAD+ NAD+ NADH NADH Pyruvate Pyruvate 5 NAD+ NADH