Electron Configuration and Lewis Dot Diagrams
advertisement

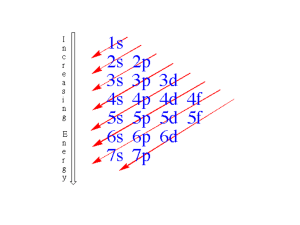
UNIT 5 CHEMICAL BONDING Chapter 6.1 Lewis Dot Diagrams & Ions STABLE ELECTRON CONFIGURATIONS When is an atom unlikely to react? When the outer shell (highest occupied energy level) is filled with electrons 8 e for Octet Rule Noble gases are the most stable elements. The highest occupied energy level is completely filled. Elements tend to react to achieve electron configurations similar to those of noble gases. STABLE ELECTRON CONFIGURATIONS Chemical properties of an element depend on the number of valence electrons. Electron dot diagram - [Lewis Dot Model] is alternative to standard electron shell diagram Dot diagram emphasizes valence electrons (inner shells hidden) ELECTRON CONFIGURATION AND LEWIS DOT DIAGRAMS F F Lewis Dot Diagram Both of these mean the same thing LEWIS DOT DIAGRAM Element symbol represents: Nucleus AND All inner core electrons Dots F represent: Valence electrons (outer shell – Highest occupied energy level) STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS STABLE ELECTRON CONFIGURATIONS – LEWIS DOT DIAGRAMS FORMATION OF IONS When an atom gains or loses an electron(s) atom is no longer neutral. # of Protons NO LONGER EQUAL # of electrons Definition: ION = atom (or group of atoms) with positive (+) or negative (-) net electric charge. FORMATION OF IONS ANION = Ion with a negative (-) charge [Memory aid: anion = A Negative ION] Example: Chlorine atomic # 17 Halogen Family/Group 7A Neutral (but not stable) - 17 protons and 17 electrons To become stable – gain 1 electron in outer e- shell 7 Valence Electrons Now have 1 more e- than proton = -1 net charge Symbol is written Cl- or Cl1- FORMATION OF IONS Naming Part Cl- Anions: of element name + suffix “ide” Example: Chlorine ion becomes “chlor” + “ide” is called a chloride ion F- is the fluoride ion (“fluor” + “ide”) Br- is the bromide ion (“brom” + “ide”) I- is the iodide ion (“iod” + “”ide”) FORMATION OF IONS CATION = Ion with a positive charge Example: Sodium atomic # 11 Alkali Metal Family/Group 1A 1 Valence Electron Neutral (but not stable) -11 protons and 11 electrons To become stable – lose the 1 electron in outer e- shell Now has 1 more proton than electron = 1+ net charge Symbol is written Na+ or Na1+ FORMATION OF IONS Naming Use Cations: the normal element name Na+ is called a sodium ion Li+ is the lithium ion Cs+ is the cesium ion Fr+ is the francium ion BONDING Elements achieve stable electron configurations by transferring or sharing electrons between atoms Transferring Electrons Those with <4 valence electrons “LEND” them These elements “lose” valence electrons OR Those with >4 valence electrons “BORROW” them These elements “gain” electrons FORMATION OF IONS & BONDING Sodium reacts with chlorine electron transferred from sodium to chlorine Each atom ends up more stable FORMATION OF IONS & BONDING Sodium reacts with chlorine electron transferred from sodium to chlorine Each atom ends up more stable FORMATION OF IONS & BONDING Sodium reacts with chlorine electron transferred from sodium to chlorine Each atom ends up more stable FORMATION OF IONS & BONDING Sodium reacts with chlorine electron transferred from sodium to chlorine Each atom ends up more stable IONIC BONDING Chemical bond = force that holds atoms or ions together as a unit. Opposites Particle(s) with negative charge attracts particle(s) with positive charge. Ionic • • attract bond Force that holds cations and anions together and which involves the transfer of electrons. Bond occurs between a metal and a nonmetal IONIC BONDING Ionic Compounds What is the chemical formula for magnesium chloride? Mg and Cl First determine the Lewis Dot Diagram and Ions for each element: Ions Mg2+ , Cl- Lewis Dot IONIC BONDING Mg atom cannot reach a stable electron configuration by giving up just 1 valence electron or reacting with just 1 chlorine atom. Mg + 2Cl OR IONIC BONDING Mg transfers 2 electrons, one to each of the 2 Cl atoms. After transfer Charge on the magnesium ion is 2+ Charge on the 2 chloride ions is 1 IONIC BONDING Formula for magnesium chloride is MgCl2 - 2+ - PROPERTIES OF IONIC COMPOUNDS The properties of sodium chloride are typical of ionic compounds. High melting point sodium chloride melts ~800°C When solid - poor conductor of electric current When liquid (melted) - good conductor of electric current. Brittle - crystals shatter when struck with a mallet EXIT SLIP Draw the Lewis Dot Diagrams for the Nitrogen Family. Provide and name the ions for Oxygen, Calcium, and Sulfur. What is the chemical formula for Aluminum Fluoride? Show the steps you used to determine the formula.