30-39
p-orbitals
S-orbitals
#30 How many electrons are in the highest occupied energy level of these atoms:
Barium
2 electrons are in the 6 th level
Sodium 1 electrons are in the 3 th level
Aluminum 3 electrons are in the 3 th level
Oxygen 6 electrons are in the 2nd level
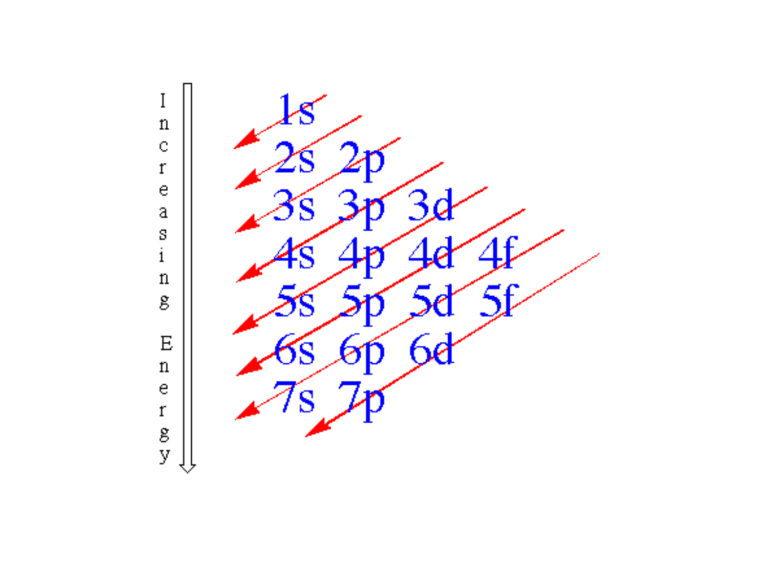
#31 aufbau principle
Pauli exclusion principle
Hund’s rule
#32
Write the electron configurations for the elements that are identified only by these atomic numbers.
15: 1s 2 2s 2 2p 6 3s 2 3p 3 phosphorous
#32
Write the electron configurations for the elements that are identified only by these atomic numbers.
12: 1s 2 2s 2 2p 6 3s 2
Magnesium
#32
Write the electron configurations for the elements that are identified only by these atomic numbers.
9: 1s 2 2s 2 2p 5 fluorine
#32
Write the electron configurations for the elements that are identified only by these atomic numbers.
18: 1s 2 2s 2 2p 6 3s 2 3p 6 argon
#33 What is meant by 3p 3 ?
3p 3
#34 Give electron configurations for atoms of these elements:
Na =11
1s 2 2s 2 2p 6 3s 1
#34 Give electron configurations for atoms of these elements:
S=16
1s 2 2s 2 2p 6 3s 2 3p 4
#34 Give electron configurations for atoms of these elements:
Mg = 12
1s 2 2s 2 2p 6 3s 2
#34 Give electron configurations for atoms of these elements:
Ne=10
1s 2 2s 2 2p 6
#34 Give electron configurations for atoms of these elements:
K=19
1s 2 2s 2 2p 6 3s 2 3p 6 4s 1
#35 Which of these orbital designations are invalid?
a. 4s b. 3f c. 2d d. 3d
#36 What is the maximum number of electrons that can go into each of the following sublevels?
a. 2s b. 3p c. 4s d. 3d
#36 e. 4p f. 5s g. 4f h. 5p
#37 Arrange the following sublevels in order of increasing energy:
3d, 2s, 4s, 3p
2s
3p
4s
3d
#38 How many electrons are in the second energy level of an atom of each element?
a. chlorine
1s 2 2s 2 2p 6 3s 2 3p 5 b. phosphorous 1s 2 2s 2 2p 6 3s 2 3p 3 c. potassium
1s 2 2s 2 2p 6 3s 2 3p 6 4s 1
#39 Write electron configuations for atoms of these elements: a. selenium
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10
4p 4
34 b. vanadium 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3 c. nickel 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8 d. calcium 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2