Arterial Blood Gas Analysis
advertisement

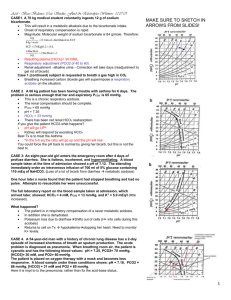
Arterial Blood Gas Analysis Overview What is an ABG? • The Components – pH / PaCO2 / PaO2 / HCO3 / O2sat / BE • Desired Ranges – – – – – – pH - 7.35 - 7.45 PaCO2 - 35-45 mmHg PaO2 - 80-100 mmHg HCO3 - 21-27 O2sat - 95-100% Base Excess - +/-2 mEq/L Why Order an ABG? • • • • Aids in establishing a diagnosis Helps guide treatment plan Aids in ventilator management Improvement in acid/base management allows for optimal function of medications • Acid/base status may alter electrolyte levels critical to patient status/care Logistics • When to order an arterial line -– Need for continuous BP monitoring – Need for multiple ABGs • Where to place -- the options – – – – – Radial Femoral Brachial Dorsalis Pedis Axillary Acid Base Balance • The body produces acids daily – 15,000 mmol CO2 – 50-100 mEq Nonvolatile acids • The lungs and kidneys attempt to maintain balance Acid Base Balance • Assessment of status via bicarbonatecarbon dioxide buffer system – CO2 + H2O <--> H2CO3 <--> HCO3- + H+ – ph = 6.10 + log ([HCO3] / [0.03 x PCO2]) The Terms • ACIDS – Acidemia – Acidosis • Respiratory CO2 • Metabolic HCO3 • BASES – Alkalemia – Alkalosis • Respiratory CO2 • Metabolic HCO3 Respiratory Acidosis • ph, CO2, Ventilation • Causes – CNS depression – Pleural disease – COPD/ARDS – Musculoskeletal disorders – Compensation for metabolic alkalosis Respiratory Acidosis • Acute vs Chronic – Acute - little kidney involvement. Buffering via titration via Hb for example • pH by 0.08 for 10mmHg in CO2 – Chronic - Renal compensation via synthesis and retention of HCO3 (Cl to balance charges hypochloremia) • pH by 0.03 for 10mmHg in CO2 Respiratory Alkalosis • pH, CO2, Ventilation • CO2 HCO3 (Cl to balance charges hyperchloremia) • Causes – – – – – Intracerebral hemorrhage Salicylate and Progesterone drug usage Anxiety lung compliance Cirrhosis of the liver Sepsis Respiratory Alkalosis • Acute vs. Chronic – Acute - HCO3 by 2 mEq/L for every 10mmHg in PCO2 – Chronic - Ratio increases to 4 mEq/L of HCO3 for every 10mmHg in PCO2 – Decreased bicarb reabsorption and decreased ammonium excretion to normalize pH Metabolic Acidosis • pH, HCO3 • 12-24 hours for complete activation of respiratory compensation • PCO2 by 1.2mmHg for every 1 mEq/L HCO3 • The degree of compensation is assessed via the Winter’s Formula PCO2 = 1.5(HCO3) +8 2 The Causes • Metabolic Gap Acidosis – – – – – – – – M - Methanol U - Uremia D - DKA P - Paraldehyde I - INH L - Lactic Acidosis E - Ehylene Glycol S - Salicylate • Non Gap Metabolic Acidosis – Hyperalimentation – Acetazolamide – RTA (Calculate urine anion gap) – Diarrhea – Pancreatic Fistula Metabolic Alkalosis • pH, HCO3 • PCO2 by 0.7 for every 1mEq/L in HCO3 • Causes – – – – – Vomiting Diuretics Chronic diarrhea Hypokalemia Renal Failure Mixed Acid-Base Disorders • Patients may have two or more acid-base disorders at one time • Delta Gap Delta HCO3 = HCO3 + Change in anion gap >24 = metabolic alkalosis The Steps • • • • Start with the pH Note the PCO2 Calculate anion gap Determine compensation Sample Problem #1 • An ill-appearing alcoholic male presents with nausea and vomiting. – ABG - 7.4 / 41 / 85 / 22 – Na- 137 / K- 3.8 / Cl- 90 / HCO3- 22 Sample Problem #1 • Anion Gap = 137 - (90 + 22) = 25 anion gap metabolic acidosis • Winters Formula = 1.5(22) + 8 2 = 39 2 compensated • Delta Gap = 25 - 10 = 15 15 + 22 = 37 metabolic alkalosis Sample Problem #2 • 22 year old female presents for attempted overdose. She has taken an unknown amount of Midol containing aspirin, cinnamedrine, and caffeine. On exam she is experiencing respiratory distress. Sample Problem #2 • ABG - 7.47 / 19 / 123 / 14 • Na- 145 / K- 3.6 / Cl- 109 / HCO3- 17 • ASA level - 38.2 mg/dL Sample Problem #2 • Anion Gap = 145 - (109 + 17) = 19 anion gap metabolic acidosis • Winters Formula = 1.5 (17) + 8 2 = 34 2 uncompensated • Delta Gap = 19 - 10 = 9 9 + 17 = 26 no metabolic alkalosis Sample Problem #3 • 47 year old male experienced crush injury at construction site. • ABG - 7.3 / 32 / 96 / 15 • Na- 135 / K-5 / Cl- 98 / HCO3- 15 / BUN- 38 / Cr- 1.7 • CK- 42, 346 Sample Problem #3 • Anion Gap = 135 - (98 + 15) = 22 anion gap metabolic acidosis • Winters Formula = 1.5 (15) + 8 2 = 30 2 compensated • Delta Gap = 22 - 10 = 12 12 + 15 = 27 mild metabolic alkalosis Sample Problem #4 • 1 month old male presents with projectile emesis x 2 days. • ABG - 7.49 / 40 / 98 / 30 • Na- 140 / K- 2.9 / Cl- 92 / HCO3- 32 Sample Problem #4 • Metabolic Alkalosis, hypochloremic • Winters Formula = 1.5 (30) + 8 2 = 53 2 uncompensated