ABG Interpretation
advertisement
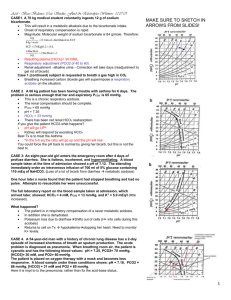
MLAB 2401: CLINICAL CHEMISTRY KERI BROPHY-MARTINEZ ABG Interpretation EVALUATING ACID-BASE DISORDERS ARMADA acid-base balance made easy in 4 steps 1. A cidosis or Alkalosis? 2. R espiratory disorder? acidosis or alkalosis check pCO2 M etabolic disorder? acidosis or alkalosis check HCO3 The one that matches the pH (acidosis or alkalosis), is the primary disorder. 3. A nion Gap? D elta AG? 4. A ssess compensation STEP 1: EVALUATE PH Evaluate pH < 7.35 = Acidosis > 7.45 = Alkalosis STEP 2: THINK “ROME” To determine whether the disorder is respiratory or metabolic use “ROME” R = Respiratory Seesawing (opposite) is pCO2 less than 35 (alkalosis) or more than 45 (acidosis) O = Opposite [pH & pCO2] M = Metabolic Swinging together o is HCO3 less than 22 (acidosis) or more than 26 (alkalosis) E = Equal [pH & HCO3] STEP 3: ANION GAP (AG) & DELTA AG Examine AG and Delta AG Anion gap = (Na + K) - (Cl + [HCO3]) (all units mmol/L) Since K is a small number, then . . . AG = Na+ - (Cl- + HCO3-) Delta AG = patient's AG - 12 mEq/L MORE ON THE ANION GAP MUDPILES Methanol Uremia of renal failure Diabetes or ketoacidosis Paraldehyde toxicity Isoniazid Lactic acidosis Ethylene glycol Salicylate STEP 4: ASSESS COMPENSATION To assess compensation compare pCO2 and HCO3- to reference range Is the pH normal? Yes Respiratory disorder compensated by kidneys Metabolic disorder compensated by lungs STEP 4: ASSESS COMPENSATION If the pH is still outside the reference range Partial compensation has occurred ACID-BASE - PROBLEM #1 pH = 7.56 pCO2 = 43 mmHG HCO3 = 38 mEQ/L pH > 7.45, then alkalosis pH & HCO3 swinging up, then Metabolic Alkalosis Since pH still high and pCO2 normal then it is Uncompensated What are some causes of Metabolic Alkalosis? ACID-BASE PROBLEM #2 pH = 7.23 PCO2 = 57 mmHG HCO3 = 23 mEQ/L pH < 7.35, then acidosis Bicarb is normal but CO2 is elevated or opposite of pH so this is Respiratory Acidosis Since the pH is still low and the HCO3 is normal it is uncompensated What causes Respiratory Acidosis? ACID-BASE PROBLEM #3 pH = 7.23 pCO2 = 45 HCO3 = 19 pH < 7.35, then acidosis pCO2 normal but HCO3 is low or swinging w/ pH so it is Metabolic Acidosis pCO2 normal so uncompensated What causes Metabolic Acidosis? ACID-BASE PROBLEM #4 pH = 7.51 pCO2 = 29 mmHG HCO3 = 20 mEq/L pH > 7.45, then alkalosis pCO2 is low or opposite pH so it is Respiratory Alkalosis Since HCO3 is low it is partially compensated What causes Respiratory Alkalosis? How is it compensated? REFERENCES Bishop, M., Fody, E., & Schoeff, l. (2010). Clinical Chemistry: Techniques, principles, Correlations. Baltimore: Wolters Kluwer Lippincott Williams & Wilkins. Carreiro-Lewandowski, E. (2008). Blood Gas Analysis and Interpretation. Denver, Colorado: Colorado Association for Continuing Medical Laboratory Education, Inc. Jarreau, P. (2005). Clinical Laboratory Science Review (3rd ed.). New Orleans, LA: LSU Health Science Center. Sunheimer, R., & Graves, L. (2010). Clinical Laboratory Chemistry. Upper Saddle River: Pearson . 13