Chemical Interactions Chapter 2 Review
advertisement

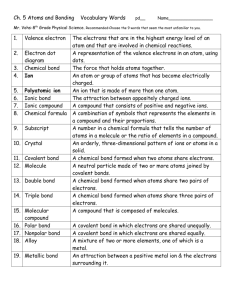
Chemical Interactions Chapter 2 Review Good Luck! How do the properties of a compound compare with the properties of the elements that make it? They are often quite different What two factors determine the properties of a compound? the types of atoms it contains and how the atoms are arranged How do we represent compounds? by a chemical formula NaNO3 has how many oxygen atoms? Three What is a subscript? a subscript is a number written to the right of a chemical symbol and slightly below it. Write the formula for one atom of nitrogen and one atom of oxygen. NO How can millions of compounds be made from the atoms of about 100 elements atoms can combine in many different ratios What do you get when an atom loses or gains an electron? an ion What do you call the force of attraction between positive and negative ions? an ionic bond What type of bond holds a crystal together? Ionic bond What is the name of MgO magnesium oxide What type of bond is formed when electrons are shared? covalent What type of bond forms from unequal sharing of electrons? Polar covalent What is responsible for many of the properties of substances? their structure What type of bond is found in individual molecules? covalent What part of an atom is involved in chemical bonding? electron cloud What property of the electrons in a metal gives the metal its properties? They move easily Are covalent compounds good conductors of electricity when dissolved? Why or Why not? No, molecules stay together and do not conduct an electrical current. How do metal atoms bond together? Metal atoms share electrons with many other metal atoms at the same time. Why do ionic compounds have high melting points? Each ion in a crystal is rigidly bonded to the several ions that surround it. Because each ion is strongly bonded to other ions, much energy has to be added to break the bonds. What are three forms of the element carbon? Graphite, diamond, and fullerene Most substances are compounds All compounds are made of atoms of two or more elements The chemical formula for a compound have one barium (Ba) ion and two chloride (Cl) ions is BaCl2 The 4 in the chemical formula CH4 means there are four hydrogen atoms to one carbon atom An atom becomes a positive ion when it loses an electron to another atom A polar covalent bond forms when two atoms share two electrons unequally Metallic bonds make many metals good conductors of electricity