4-3 Power Point
advertisement
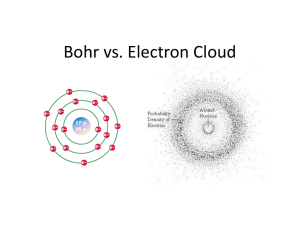
4.3 Modern Atomic Theory 4-3 Cornell • Bohr’s Model of the Atom – Energy Levels – Draw an example of Bohr’s Model – Evidence of energy levels • What happens when they change energy levels – Electron Cloud Model • Draw a picture of it. • How is it different than Bohr’s Model. – Atomic Orbitals – Electron Configurations 4.3 Modern Atomic Theory Have you ever wondered what produces the different colors in a fireworks display? Certain compounds will produce certain colors of light when they are heated. Compounds containing the element strontium produce red light. Compounds containing barium produce green light. 4.3 Modern Atomic Theory Bohr’s Model of the Atom As did Rutherford's atomic model, Bohr’s atomic model had a nucleus surrounded by a large volume of space. But Bohr’s model focused on the electrons and their arrangement. In Bohr’s model, electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Each electron in an atom has a specific amount of energy. 4.3 Modern Atomic Theory III. Modern Atomic Theory A. Bohr’s Model of the Atom 1. Energy levels- The possible energies that electrons in an atom can have. a. An electron in an atom can move from one energy level to another when the atom gains or loses energy. Electron Electrons gain or lose energy when they move between fixed energy levels Nucleus Bohr Model 4.3 Modern Atomic Theory Bohr’s Model of the Atom An analogy for energy levels of electrons is a staircase. • The landing at the bottom of the staircase is the lowest level. • Each step up represents a higher energy level. • The step height represents an energy difference between levels. • You can only move in whole numbers of stairs. 4.3 Modern Atomic Theory b. An electron may move up or down two or more energy levels if it gains or loses the right amount of energy. c. The size of the jump between energy levels determines the amount of energy gained or lost. d. An electron cannot exist between energy levels. e. No two elements have the same set of energy levels. 4.3 Modern Atomic Theory 2. Evidence for Energy Levels a. Scientists can measure the energy gained when electrons absorb energy and move to a higher energy level b. Scientist can measure the energy released when the electron moves to a lower energy level. c. Light is a form of energy that can be observed. 4.3 Modern Atomic Theory Bohr’s Model of the Atom The movement of electrons between energy levels explains the light you see when fireworks explode. • Heat causes some electrons to move to higher energy levels. • When those electrons move back to lower energy levels, they release energy. Some of that energy is released as visible light. • Different elements emit different colors of light because no two elements have the same set of energy levels. 4.3 Modern Atomic Theory Electron Cloud Model Bohr’s model was improved as scientists made further discoveries. Bohr correctly assigned energy levels to electrons, but electrons do not move like planets in a solar system. 4.3 Modern Atomic Theory B. Electron Cloud- a visual model of the most likely locations for electrons in an atom 1. Today, scientists use probability when trying to predict the locations and motions of electrons in atoms 2. Scientists use the electron cloud model to describe the possible locations of electrons around the nucleus. 4.3 Modern Atomic Theory Electron Cloud Model The electron cloud model replaced Bohr's vision of electrons moving in predictable paths. The electron cloud is a visual model of the probable locations of electrons in an atom. The probability of finding an electron is higher in the denser regions of the cloud. The nucleus contains protons and neutrons Electron Cloud Model 4.3 Modern Atomic Theory Electron Cloud Model The photographs below provide an analogy for an electron cloud. When the propeller of an airplane is at rest, you can see the location of the blades. When the propeller is moving, you see only a blur that is similar to a drawing of an electron cloud. 4.3 Modern Atomic Theory C. Atomic Orbitals- An orbital is a region of space around the nucleus where an electron is likely to be found. 1. The electron cloud represents all the orbitals in an atom 2. An electron cloud is a good approximation of how electrons behave in their orbitals. 4.3 Modern Atomic Theory Electron Cloud Model For an analogy to the concept of an orbital, imagine a map of your school. Mark your exact location with a dot once every 10 minutes over a period of one week. The dots on your map are a model of your “orbital.” They describe your most likely locations. • The places you visit the most would have the highest concentration of dots. • The places you visit the least would have the lowest concentration of dots. 4.3 Modern Atomic Theory 3. The level in which an electron has the least energy—the lowest energy level—has only one orbital. 4. Higher energy levels have more than one orbital. 4.3 Modern Atomic Theory D. Electron Configurations- the arrangement of electrons in the orbitals of an atom 1. When all the electrons in an atom have the lowest possible energies, the atom is said to be in its ground state. 2. The most stable electron configuration is the one in which the electrons are in orbitals with the lowest possible energies. 4.3 Modern Atomic Theory Electron Configurations A lithium atom has three electrons. • In the ground state, two of the lithium electrons are in the orbital of the first energy level. • The third electron is in an orbital of the second energy level. • If a lithium atom absorbs enough energy, one of its electrons can move to an orbital with a higher energy. • This configuration is referred to as an excited state. An excited state is less stable than the ground state. • Eventually, the electron that was promoted to a higher energy level loses energy, and the atom returns to the ground state. 4.3 Modern Atomic Theory Electron Configurations The ground state of a person is on the floor. A gymnast on a balance beam is like an atom in an excited state—not very stable. When she dismounts, the gymnast will return to a lower, more stable energy level. 4.3 Modern Atomic Theory Assessment Questions 1. According to Bohr’s model of the atom, which of the following can happen when an atom gains energy? a. b. c. d. An atom returns to its ground state. A neutron can be changed into a proton. A proton can move to a higher energy level. An electron can move to a higher energy level. 4.3 Modern Atomic Theory Assessment Questions 1. According to Bohr’s model of the atom, which of the following can happen when an atom gains energy? a. b. c. d. An atom returns to its ground state. A neutron can be changed into a proton. A proton can move to a higher energy level. An electron can move to a higher energy level. ANS: D 4.3 Modern Atomic Theory Assessment Questions 2. How does the modern atomic theory describe the location of electrons in an atom? a. Electrons move randomly in space around the nucleus. b. Electrons can be described as a cloud based on probable locations. c. Electrons orbit the nucleus in the same way that planets orbit the sun. d. Electrons move in a spiral pattern if increasing distance from the nucleus. 4.3 Modern Atomic Theory Assessment Questions 2. How does the modern atomic theory describe the location of electrons in an atom? a. Electrons move randomly in space around the nucleus. b. Electrons can be described as a cloud based on probable locations. c. Electrons orbit the nucleus in the same way that planets orbit the sun. d. Electrons move in a spiral pattern if increasing distance from the nucleus. ANS: B 4.3 Modern Atomic Theory Assessment Questions 3. What is meant when an atom is said to be in its ground state? a. There is no net charge on the atom. b. The number of protons equals the number of neutrons. c. The atom’s electrons all have the lowest possible energies. d. It is the isotope with the least number of neutrons. 4.3 Modern Atomic Theory Assessment Questions 3. What is meant when an atom is said to be in its ground state? a. There is no net charge on the atom. b. The number of protons equals the number of neutrons. c. The atom’s electrons all have the lowest possible energies. d. It is the isotope with the least number of neutrons. ANS: C