CHEM II Periodic Trends
advertisement

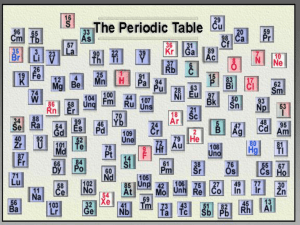
Trends in the Periodic Table Periodic Trends In this chapter, we will rationalize observed trends in Sizes of atoms and ions. Ionization energy. Electron affinity. Why is the Periodic Table important to me? The periodic table is the most useful tool to a chemist. You get to use it on every test. It organizes lots of information about all the known elements. PERIODIC LAW When elements are arranged in order of increasing atom number, their physical and chemical properties show a periodic pattern HISTORY OF FORMATION OF PERIODIC TABLE ANTOINE LAVOISIER Late 1790’s French Chemist Compiled a list of 23 known elements. Many of the elements were known to exist since prehistoric times (including Au, Ag, C, O) HISTORY OF FORMATION OF PERIODIC TABLE By mid-1800’s the known elements was approximately 70. The discovery of electricity, spectrometer, and the onset of the industrial revolution led to this explosion in the number of elements. HISTORY OF FORMATION OF PERIODIC TABLE DMITRI MENDELEEV 1869 Russian Chemist First real periodic table Combined both Doberiener and Newlands Dmitri Mendeleev: Father of the Table HOW HIS WORKED… Put elements in rows by increasing atomic weight. Put elements in columns by the way they reacted. SOME PROBLEMS… He left blank spaces for what he said were undiscovered elements. (Turned out he was right!) He broke the pattern of increasing atomic weight to keep similar reacting elements together. Medeleev’s Table The Current Periodic Table Mendeleev wasn’t too far off. Now the elements are put in rows by increasing ATOMIC NUMBER!! The horizontal rows are called periods and are labeled from 1 to 7. The vertical columns are called groups are labeled from 1 to 18. Families on the Periodic Table Columns are also grouped into families. Families may be one column, or several columns put together. Families have names rather than numbers. (Just like your family has a common last name.) Hydrogen Hydrogen belongs to a family of its own. Hydrogen is a diatomic, reactive gas. Hydrogen was involved in the explosion of the Hindenberg. Hydrogen is promising as an alternative fuel source for automobiles Groups of Elements 1 1 1 2 3 Be 3 4 K 7 Oxygen group or Chalcogen Transition metals 17 Halogens 13 Boron group 18 Noble gases 14 Carbon group 3 5 V 14 C 15 N 16 O 5 6 7 8 9 10 Al Si P S Cl Ar 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 Hf Ta W 72 73 74 Cs Ba 55 56 Fr Ra 87 88 * W 25 43 26 44 Re Os 75 76 27 28 29 47 30 45 46 Ir Pt Au Hg Tl 77 78 81 79 48 31 80 32 33 34 Sn Sb Te 50 51 Pb Bi 82 83 52 Kr 35 36 I Xe 53 54 Po At Rn 84 85 86 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 W 24 18 Rf Db Sg Bh Hs Mt 104 * 17 2 F Ne 8 6 7 9 10 11 12 13 14 15 16 17 Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 21 38 23 13 B Hydrogen Inner transition metals 4 Ca Sc Ti 12 18 He 20 37 6 16 Na Mg 19 5 Alkali earth metals 2 Li 11 4 Nitrogen group 2 H 1 15 Alkali metals 58 59 Ac Th Pa 89 90 91 60 U 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103 Groups of Elements 1 18 H 1 He 2 13 14 15 16 17 2 Li Be N O F Ne 3 4 7 8 9 10 Na Mg P S Cl Ar 11 12 15 16 17 18 K Ca As Se Br Kr 19 20 33 34 35 36 Rb Sr Sb Te I Xe 37 38 51 52 53 54 Cs Ba Bi Po At Rn 55 56 83 84 85 86 Fr Ra 87 88 1 Alkali metals 16 Oxygen family or Chalocogen 2 Alkaline earth metals 17 Halogens 18 Noble gases 15 Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 367 Nitrogen family Alkaline Earth Metals Second column on the periodic table. (Group 2) Reactive metals that are always combined with nonmetals in nature. Several of these elements are important mineral nutrients (such as Mg and Ca GROUPS OF ELEMENTS IN PERIODIC TABLE METALS Vast majority of elements are metals. Elements found to the left of the heavy black line on periodic table. Properties that most metals exhibit: GROUPS OF ELEMENTS IN PERIODIC TABLE METALS (cont.) Physical Properties: Appearance – high luster Tapping – malleable Conductivity – conductor of heat and electricity GROUPS OF ELEMENTS IN PERIODIC TABLE METALS (cont.) Chemical Properties: Tend to form positive ions In HCl – gas produced; corrosion In CuCl2 - corrosion Metallic Trends in Periodic Table metallic character increases nonmetallic character increases metallic character increases nonmetallic character increases Transition Metals Elements in groups 312 Less reactive harder metals Includes metals used in jewelry and construction. Metals used “as metal.” TRENDS IN PERIODIC TABLE ATOMIC RADII Definition - distance from nucleus to outer edge of electron cloud of atom Trend from left to right – decreases Rationale – increasing nuclear charge Trend from top to bottom – increases Rationale – increasing energy levels Carbon Family Elements in group 14 Contains elements important to life and computers. Carbon is the basis for an entire branch of chemistry. Silicon and Germanium are important semiconductors. Nitrogen Family Elements in group 15 Nitrogen makes up over ¾ of the atmosphere. Nitrogen and phosphorus are both important in living things. Most of the world’s nitrogen is not available to living things. The red stuff on the tip of matches is Oxygen Family or Chalcogens Elements in group 16 Oxygen is necessary for respiration. Many things that stink, contain sulfur (rotten eggs, garlic, skunks,etc.) Halogens Elements in group 17 Very reactive, volatile, diatomic, nonmetals Always found combined with other element in nature . Used as disinfectants and to strengthen teeth. GROUPS OF ELEMENTS IN PERIODIC TABLE NONMETALS Second most amount of elements. Elements found to the right of the heavy black line on periodic table. Properties that most nonmetals exhibit: GROUPS OF ELEMENTS IN PERIODIC TABLE NONMETALS (cont.) Chemical Properties: Tends to form negative ions In HCl – no reaction In CuCl2 – no reaction GROUPS OF ELEMENTS IN PERIODIC TABLE METALLOIDS Elements that straddle the heavy black line Some discrepancy in number(6,7, or 8) Combination of metallic and nonmetallic properties. GROUPS OF ELEMENTS IN PERIODIC TABLE NOBLE GASES Elements found in group 18. Extremely unreactive elements. The Noble Gases The Noble Gases Elements in group 18 VERY unreactive, monatomic gases Used in lighted “neon” signs Used in blimps to fix the Hindenberg problem. Have a full valence shell. TRENDS IN PERIODIC TABLE PHYSICAL PROPERTIES (cont.) Boiling Point Left to Right – increase through metals then decreases through nonmetals Top to Bottom – decreases in metals and increase in nonmetals TRENDS IN PERIODIC TABLE REACTIVITY (cont.) Nonmetals Trend from left to right – increase Trend from top to bottom – decrease TRENDS IN PERIODIC TABLE REACTIVITY Metals Trend from left to right – decrease Trend from top to bottom – increase Factors Affecting Chemical Trends Nuclear Charge – strength of the nucleus The larger the nuclear charge, the stronger the pull on the last electron Sublevel Stability An electron from a full or half-full sublevel requires additional energy to be removed. Shielding effect – electrons between nucleus and outer electron The greater the shielding effect, the less the pull on the last electron. Radius – distance from nucleus to outer electron The greater the distance between the nucleus and the outer electrons of an atom, the less the pull on the last electron. Smoot, Price, Smith, Chemistry A Modern Course 1987, page 189 Periodic Trends in Atomic Radii LeMay Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 175 TRENDS IN PERIODIC TABLE IONIC RADII Definition – distance from nucleus to outer edge of ion Trend for more positive ion – decreases Rationale – increasing relative nuclear charge Trend for more negative ion – increases Rationale – decreasing relative nuclear charge ANIONS BIGGER THAN CATIONS IN A GIVEN PERIOD!!!! Periodic Trends in Ionic Radii IONIC RADII 1 2 Li1+ Be2+ 0.60 Na1+ 0.95 K1+ 1.33 3 0.65 Ca2+ 0.99 Al3+ 0.50 Ga3+ 0.62 In3+ Rb1+ 1.48 Sr2+ 1.13 6 N3- O2- 1.71 0.31 Mg2+ 5 0.81 1.40 S21.84 7 F11.36 Cl11.81 Br1- Se21.98 1.85 I1- Te22.21 2.16 Tl3+ Cs1+ 1.69 Ba2+ 1.35 0.95 = 1 Angstrom Trends in Atomic and Ionic Size Metals Nonmetals Group 1 Group 13 Group 17 e e Li+ Li 152 F 64 60 e e Na+ Na 95 e e 136 e Al3+ Al 143 F1- 50 Cl Cl1- 99 186 181 e e K+ Br K 227 133 Cations are smaller than parent atoms 114 Br-1195 Anions are larger than parent atoms e Li+ Li 152 60 e e Li+ e Li Li + e Lithium ion Lithium atom Lithium atom 60 Lithium ion TRENDS IN PERIODIC TABLE FIRST IONIZATION ENERGY Definition – amount energy needed to remove the outer most electron Trend from left to right – increases Rationale – increased nuclear charge Trend from top to bottom – decreases Rationale – increased energy levels and shielding effect TRENDS IN PERIODIC TABLE ELECTRON AFFINITY Definition – the attraction of an atom for an electron Trend from left to right – increases Rationale – increasing nuclear charge Trend from top to bottom – decreases Rationale – increasing energy levels and shielding effect TRENDS IN PERIODIC TABLE ELECTRONEGATIVITY Definition – the tendency of an atom to attract electrons to itself when it is combined with another atom Trend from left to right – increases Trend from top to bottom – decreases Nuclear charge increases Shielding increases Atomic radius increases Ionic size increases Ionization energy decreases Electronegativity decreases Summary of Periodic Trends Shielding is constant Atomic radius decreases Ionization energy increases Electronegativity increases Nuclear charge increases 1A 0 2A Ionic size (cations) decreases 3A 4A 5A 6A 7A Ionic size (anions) increases Electronegativities 1A 1 Period 2 3 4 5 6 7 8A H 2.1 2A 3A 4A 5A 6A 7A Li Be B C N O F 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Al Si P S Cl 1.5 1.8 2.1 2.5 3.0 Na Mg 1.2 3B 4B 5B 6B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0.8 1.0 1.3 1.5 1.6 1.6 1.7 1.6 1.8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 0.8 1.2 1.4 1.6 1.8 1.9 2.2 2.2 2.2 1.7 1.7 1.8 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0.7 1.1 1.3 1.5 1.7 1.9 2.2 2.2 1.8 1.8 2.0 1.0 0.9 y Fr Ra Ac 0.7 0.9 1.1 8B 7B 1.5 1.8 2.2 1.8 1B 2B 0.9 1.8 1.9 1.9 2.4 1.9 2.0 1.9 1.9 2.4 2.1 * Lanthanides: 1.1 - 1.3 yActinides: 1.3 - 1.5 Hill, Petrucci, General Chemistry An Integrated Approach 2nd Edition, page 373 Below 1.0 2.0 - 2.4 1.0 - 1.4 2.5 - 2.9 1.5 - 1.9 3.0 - 4.0 2.8 I 2.5 2.2