What can the periodic table tell us?
advertisement
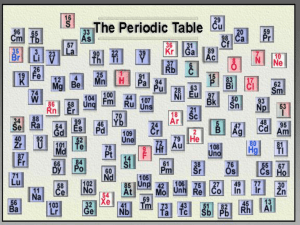
4-15a: Through gaining an understanding of the structure of atoms and how they join, I can begin to connect the properties of substances with their possible structures. Collect a learning outcomes sheet and stick this into your jotter What does this show us? Remember that all the elements have been given their own symbol to help us identify them. Every element also has its own number. This is known as its atomic number. Going through the periodic table the atomic number of the elements increase by one each time. Use the periodic table in your data book to complete the table in activity 1. Stuck? Ask for the help sheet Element Name Symbol Atomic Number Hydrogen Carbon S Br 20 80 Al K Lead 92 The Periodic Table above shows most of the elements that have been discovered. But why does the Periodic Table have this funny shape? This can be explained by looking at the work of a famous Russian chemist called Dmitri Mendeleev. He was born in 1834 and in 1869 he came up with the shape of the periodic table despite only knowing about 56 elements, clever stuff! So how did he do it? Dmitri knew how heavy the elements were and he also knew their chemical properties (how they reacted) and he noticed that some elements had similar reactions. Activity 2 My observations… Activity 3 You are going to complete a task similar to that of Dmitri to see if you can come up with the same shape. 1. Each card has an element symbol and atomic number on it. Elements of the same colour undergo similar chemical reactions. 2. In your group try and arrange the elements in atomic number order, keeping elements that undergo similar chemical reactions together. 3. Be careful, some elements may be missing. Based on what you have found out answer the following questions: What do you notice about the order of the elements in the periodic table? What do you notice about elements in the same column in the periodic table? What do you notice about the order of the elements in the periodic table? The elements are arranged in order of increasing atomic number What do you notice about elements in the same column in the periodic table? Element in the same column of the periodic table have similar chemical properties A column in the periodic table is known as a group and a row is known as a period. This is because each period shows a repeating pattern of the one before it. Activity 4 Stick in the periodic table and add labels to show the names of different parts of the periodic table as listed next. Some of the groups in the periodic table have been given names. The group 1 elements are known as the alkali metals. Group 2 elements are called the alkali earth metals. Group 7 elements are known as the halogens. Group 8 (group 0) are called the noble gases. The block in the middle between groups 2 and 3 are known as the transition metals. Can you remember where the metals and non-metals are on the periodic table? Mark on the zigzag line that separates the metals and non-metals. What Dmitri Mendeleev made as the Periodic Table is not the only way we have to arrange the elements. In fact some scientists think that a round Periodic Table is a better way to arrange the elements. Activity 4b Collect a sheet for the activity and follow the instructions carefully. Which way of arranging the elements do you prefer?