atomic
advertisement

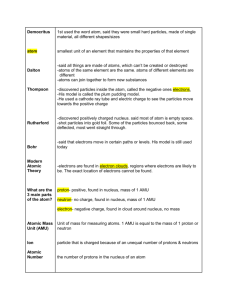
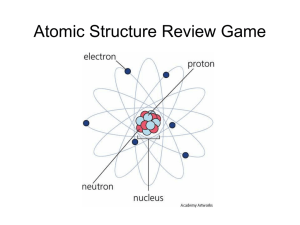
Chapter 5 Sections 2 and 3 Structure of an atom nucleus- center of an atom -discovered by Ernest Rutherford in 1911 proton (p+) -positively charged subatomic particle -found in the nucleus of an atom -discovered by Eugen Goldstein in 1886 -mass= 1.67 X 10-24g neutron (n0) -subatomic particle with no charge -found in the nucleus -discovered by James Chadwick in 1932 -mass= 1.67 X 10-24g electron (e-) -negatively charged subatomic particle -found surrounding the nucleus in energy levels or electron clouds -discovered by JJ Thomson in 1897 -mass= 9.11 X 10-28g *atoms are electrically neutral because: # of p+ = # of e-most of the mass is in the nucleus of that atom atomic number- number of protons in the nucleus of an atom (smaller number on the periodic table) -also = # of electrons b/c of above * mass number- total number of protons and neutrons in an atom -larger number on periodic table -must be rounded to a whole number # of n0 = mass # - atomic # -element can be written as He-4 isotope- atoms of the same element having the same # of p+ and e-, but different # of n0. exNe-20 10p+ 10e- 10n0 Ne-21 10p+ 10e- 11n0 Ne-22 10p+ 10e- 12n0 atomic mass- average mass of all the isotopes of an element -bigger number on periodic table unrounded -units are amu (atomic mass unit) relative abundance- which isotope is more abundant -is a % -the element that is most abundant is the one that has a mass closest to the atomic mass exCu-63 or Cu-65 Which is most abundant? Cu-63 b/c its mass is closer to atomic mass