Clausius-Clapeyron Equation
advertisement

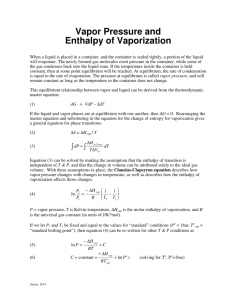
Clausius-Clapeyron Equation As assigned by Mr. Amendola despite the fact that he is no longer our chemistry teacher The Men Behind the Equation Rudolph Clausius German physicist and mathematician One of the foremost contributors to the science of thermodynamics Introduced the idea of entropy Significantly impacted the fields of kinetic theory of gases and electricity Benoit Paul Émile Clapeyron French physicist and engineer Considered a founder of thermodynamics Contributed to the study of perfect gases and the equilibrium of homogenous solids The Equation In its most useful form for our purposes: P1 H vap 1 1 ln ( ) P2 R T2 T1 In which: P1 and P2 are the vapor pressures at T1 and T2 respectively T is given in units Kelvin ln is the natural log R is the gas constant (8.314 J/K mol) ∆Hvap is the molar heat of vaporization Deriving the Equation We will use this diagram in deriving the ClausiusClapeyron equation. This diagram represents a generalized phase diagram. The line acts as a phase line, or a coexistent curve, separating phases α and β. As we know, this indicates that at all points on the line, phases α and β are in equilibrium. Deriving the Equation Since the phases are in equilibrium along the line, ∆G=0 ∆G=∆H-T∆S Since ∆G=0, ∆S=∆H/T We want to find the slope of the coexistent curve. However, since the graph we are examining is a curve rather than a line, the slope must be found by using calculus. The slope is represented by dy/dx, or in the case of the phase diagram, dp/dt. To represent the derivative along the coexistent curve, we write: p ( ) G T The curved “d” represents the use of a partial derivative. Deriving the Equation We use the cyclic rule, a rule of calculus, to find: We previously wrote ∆G=∆H-T∆S. We can also represent this as ∆G=P∆VT∆S (see the thermodynamics chapter of your book). Taking the derivative of both sides, we find that d∆G=∆VdP-∆SdT We have two variables in this differential equation: T and P. To solve this, we treat this in two cases. First, we consider P as a constant. Then, we consider T a constant. By manipulation, we find: Deriving the Equation Substituting in the equation we found through the cyclic rule, we find: As ∆S=∆H/T, this can be written as: We integrate this equation, assuming ∆H and ∆V to be constant, to find: Useful Information The Clausius-Clapeyron models the change in vapor pressure as a function of time The equation can be used to model any phase transition (liquid-gas, gas-solid, solid-liquid) Another useful form of the ClausiusClapeyron equation is: ln P H vap RT C Useful Information We can see from this form that the Clausius-Clapeyron equation depicts a line ln P H vap RT C Can be written as: H vap 1 ln P C R T which clearly resembles the model y=mx+b, with ln P representing y, C representing b, 1/T acting as x, and -∆Hvap/R serving as m. Therefore, the Clausius-Clapeyron models a linear equation when the natural log of the vapor pressure is plotted against 1/T, where -∆Hvap/R is the slope of the line and C is the y-intercept Useful Information ln P H vap RT C We can easily manipulate this equation to arrive at the more familiar form of the equation. We write this equation for two different temperatures: H vap H vap ln P C ln P1 C 2 RT2 RT1 Subtracting these two equations, we find: ln P1 ln P2 H vap H vap H vap 1 1 RT1 R2 R T2 T1 Common Applications Calculate the vapor pressure of a liquid at any temperature (with known vapor pressure at a given temperature and known heat of vaporization) Calculate the heat of a phase change Calculate the boiling point of a liquid at a nonstandard pressure Reconstruct a phase diagram Determine if a phase change will occur under certain circumstances An example of a phase diagram Real World Applications Chemical engineering Determining the vapor pressure of a substance Meteorology Estimate the effect of temperature on vapor pressure Important because water vapor is a greenhouse gas Shortcomings The Clausius-Clapeyron can only give estimations We assume changes in the heat of vaporization due to temperature are negligible and therefore treat the heat of vaporization as constant In reality, the heat of vaporization does indeed vary slightly with temperature