Energetics II - Miller, Jonathan

Energetics
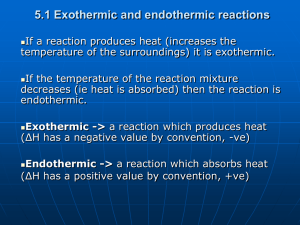
(a) Explain that some chemical reactions are accompanied by energy changes, principally in the form of heat energy; the energy changes can be exothermic (
D
H, negative) or endothermic
(b) Explain and use the terms:
(i) Enthalpy change of reaction and standard conditions, with particular reference to: formation, combustion, hydration, solution, neutralisation, atomisation
(ii) Bond energy (
D
H positive, i.e. bond breaking)
(c)
(d)
Calculate enthalpy changes from appropriate experimental results, including the use of the relationship
Enthalpy change = mc
D
T
Apply Hess’ Law to construct simple energy cycles, and carry out calculations involving such cycles and relevant energy terms, with particular reference to:
(i) determining enthalpy changes that cannot be found by direct experiment, e.g. an enthalpy change of formation from enthalpy changes of combustion
(ii) average bond energies
(e) Construct and interpret a reaction pathway diagram, in terms of the enthalpy change of the reaction and of the activation energy
Direction of heat flow
(Energy accompanies all reactions)
Endothermic reactions require energy to form products (∆H is positive)
Exothermic reactions release energy as a product (∆H is negative)
Endothermic and exothermic processes
Endothermic:
• Melting
• Vaporizing
• Chemical reactions that have a positive
∆H (absorb energy)
Exothermic
• Freezing
• Condensing
• Combustion
• Chemical reactions that have a negative
∆H (release energy)
Calculating the enthalpy (
D
H ) change of a reaction
D
H = m x c x
D
T
The specific heat capacity, c is the amount of heat needed to raise the temperature of 1 g of substance by 1 K. Its units are joules per gram per Kelvin, or Jg -1 K -1 .
For example, the specific heat capacity of water is 4.2 g -1 K -1 , so it takes
4.2 joules to raise the temperature of 1 gram of water by 1 degree kelvin.
Hess’s Law states that the total energy (or enthalpy) for a chemical reaction is the same, whatever route is taken, provided that the initial and final conditions are the same.
We can show this on a diagram called a thermochemical cycle
See book, 13.5.2 for calculations (p.132)
The standard molar enthalpy change of formation ,
D
H f is the enthalpy change when a mole of compound is formed from its elements in their standard states under standard conditions
The standard molar enthalpy of combustion
D
H c is the enthalpy change at standard state when a mole of substance is completely burned in oxygen