26.1 Properties of nucleus
advertisement
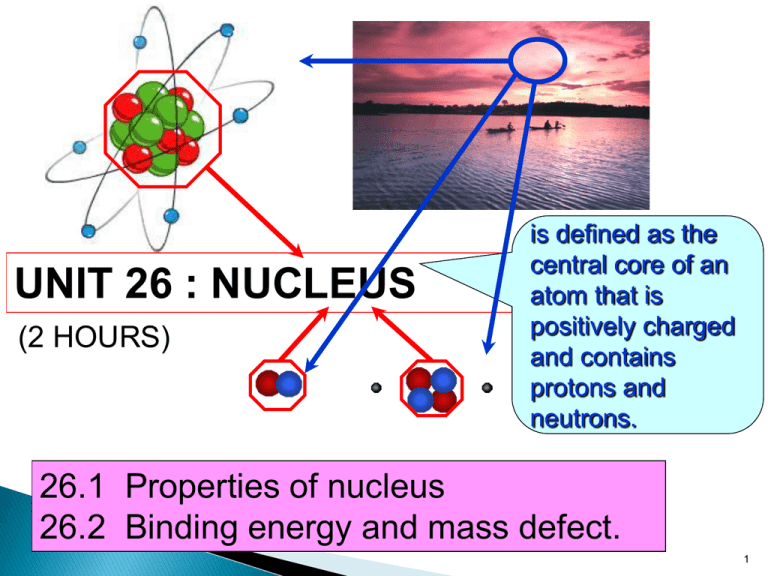
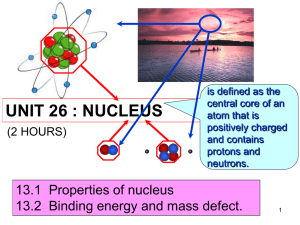
UNIT 26 : NUCLEUS (2 HOURS) is defined as the central core of an atom that is positively charged and contains protons and neutrons. 26.1 Properties of nucleus 26.2 Binding energy and mass defect. 1 At the end of this topic, students should be able to: State the properties of proton and neutron Define ◦ Proton number ◦ Nucleon number ◦ Isotopes Use to represent a nuclide 2 26.1 Properties of nucleus • A nucleus of an atom is made up of protons and neutrons that is also known as nucleons. Figure 26.1.1( atom) Figure 26.1.2 (nucleus) 3 Proton Particle with positive charge of the nucleus Charge : +1.60 x 10-19 C Mass : 1.672 x 10-27 kg / 1.007276 u Neutron Particle with no charge of the nucleus Charge : Mass : 1.675 x 10-27 kg / 1.008665 u 4 Proton number Definition: the number of protons in the nucleus. Also called as atomic number Symbol : Z Nucleon number Definition : the total number of neutrons and protons in the nucleus. Also called as atomic mass number Symbol : A Isotope Definition : the atoms of the same element whose nuclei contain the same number of protons (Z) but different number of neutrons (N). 1 2 3 Example : 1 H , 1 H , 1 H (Hydrogen, deuterium, tritium) 5 The atomic nucleus can be represented as A Z where X X = symbol for the element Z = atomic number (number of protons) A = atomic mass number = total number of protons and neutrons Example : 56 26 Fe Element : Iron-56 Proton no, Z = 26 Nucleon no, A = 56 Neutron = 56-26 = 30 A-Z=N 6 Example 26.1 Complete the table below: Element nuclide 1 1 9 4 Number of neutrons Number of electrons H Be 14 7 N 16 8 O 23 11 59 27 Na 31 16 133 55 Number of protons 8 8 8 Co S Cs 238 92 U 7 At the end of this topic, students should be able to: Define and determine mass defect Define and determine binding energy, Identify the average value of binding energy per nucleon of stable nuclei from the graph of binding energy per nucleon against nucleon number. 8 Definition the difference between the sum of the masses of individual nucleons that form an atomic nucleus and the mass of the nucleus. Formula Δ m Zm p Nm n M A m p : mass of a proton M A mass of a nucleus m n : mass of a neutron Z number of protons N number of neutrons 9 Example 26.2 From example above, can you determine the value of mass defect ? (Ans : 0.040475 a.m.u) 10 Definition Energy required to separate a nucleus into its individual protons and neutrons. @ Energy released when nucleus is formed from its individual nucleons. Formula Where E : Binding energy Δm : Mass defect c : speed of light = 3.00 x 108ms-1 11 There are 2 methods to determine the value of Binding Energy, EB EB ( in unit MeV ) Δm ( in unit u ) EB ( in unit J ) Δm ( in unit kg ) c = 3.00 x 108ms-1 2 c = 931.5 M eV u Example : Let Δm = 1 u = 1.66 x 10-27kg E B = Δmc 2 = (1.66× 10 E B = Δmc -27 8 -1 2 kgm s 9 3 1 .5 M eV = (1 u ) u J = 9 3 1 .5 M eV kg)(3.00× 10 m s ) = 1.4904 × 10 -10 = 1.4904 × 10 -10 2 2 -2 = Note : 1eV = 1.6 x 10-19J 12 Example 26.3 Calculate a) mass defect and b) binding energy of the deuterium. 2 Given H m ass 2 .0 1 3 5 5 3 u 1 Solution: 13 Example 26.4 4 2 Calculate binding energy of the Helium nucleus, H e in SI unit. Given mass of helium atom = 4.002603 u Solution: 14 EB N Definition mean (average) binding energy of a nucleus Binding energy per nucleon Binding energy ( EB ) Nucleon number( A) Binding energy per nucleon mc 2 A Binding energy per nucleon is measure the stability of of the nucleus. The greater the binding energy per nucleon, the more stable the nucleus is. 15 Binding energy per nucleon (MeV/nucleon) Greatest stability Binding energy per nucleon as a function of mass number,A Mass number A 16 From the graph: For light nuclei the value of EB/A rises rapidly from 1 MeV/nucleon to 8 MeV/nucleon with increasing mass number A. For the nuclei with A between 50 and 80, the value of EB/A ranges between 8.0 and 8.9 Mev/nucleon. The nuclei in these range are very stable. 62 The nuclide 28 Ni has the largest binding energy per nucleon (8.7945 MeV/nucleon). For nuclei with A > 62, the values of EB/A decreases slowly, indicating that the nucleons are on average, less tightly bound. For heavy nuclei with A between 200 to 240, the binding energy is between 7.5 and 8.0 MeV/nucleon.These nuclei are unstable and radioactive. 17 Example 26.5 Calculate the average binding energy per nucleon of the iron-56 56 Fe . Given 26 56 26 1 1 Fe mass 55.93494 u H 1 p mass 1.00782 u 1 0 1 n mass 1.00867 u Solution: 18 19 Exercise 20 1) The binding energy of the neon 10 Ne is160.64 MeV. Find its atomic mass. Given 1 p mass 1.007825 u 1 1 0 n mass 1.008665 u (Ans: 19.992u) 2) Determine the total binding energy and the binding energy per nucleon for the nitrogen -14 nucleus 14 N 7 Given 14 7 1 1 N mass 14.003074 u H 1 p mass 1.007825 1 0 1 n mass 1.008665 u u (Ans:104.6 MeV,7.47 MeV/nucleon) 20 3) Calculate the binding energy of an aluminum nucleus in MeV. (Given mass of neutron, mn=1.00867 u ; mass of proton, mp=1.00782 u ; speed of light in vacuum, c=3.00108 m s1 and atomic mass of aluminum, MAl=26.98154 u) (Ans: 225 MeV) 4) Calculate the binding energy per nucleon of a boron nucleus in J/nucleon. (Given mass of neutron, mn=1.00867 u ; mass of proton, mp=1.00782 u ; speed of light in vacuum, c=3.00108 m s1 and atomic mass of boron, MB=10.01294 u) (E = 1.04x10 -12 J/nucleon) 21 5) Why is the uranium-238 nucleus is less stable than carbon12 nucleus? Give an explanation by referring to the binding energy per nucleon. (Given mass of neutron, mn=1.00867 u ; mass of proton, mp=1.00782 u ; speed of light in vacuum, c=3.00108 m s1; atomic mass of carbon-12, MC=12.00000 u and atomic mass of uranium-238, MU=238.05079 u ) (Ans: U think) The end….. Next chapter : nuclear reaction 22