A nucleus
advertisement
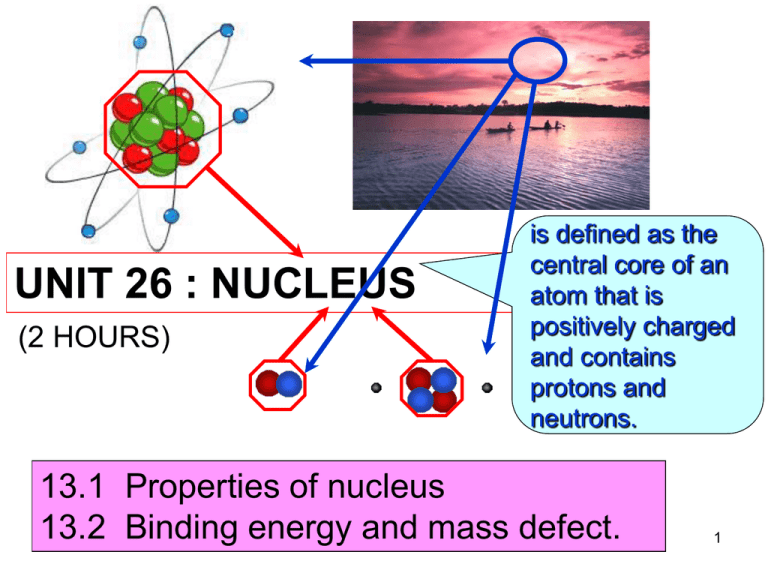
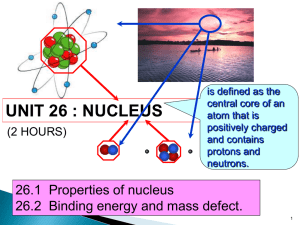
UNIT 26 : NUCLEUS (2 HOURS) is defined as the central core of an atom that is positively charged and contains protons and neutrons. 13.1 Properties of nucleus 13.2 Binding energy and mass defect. 1 26.1 Properties of nucleus (1/2 Hour) At the end of this topic, students should be able to: • State the properties of proton and neutron • Define – Proton number – Nucleon number – Isotopes • Use to represent a nuclide 2 26.1 Properties of nucleus • A nucleus of an atom is made up of protons and neutrons that is also known as nucleons. ~10-15 m A nucleus 3 13.1 Properties of nucleus Properties of proton and neutron. particle mass (kg) Proton,p 1.672 x 10-27 Neutron,n 1.675 x 10-27 charge (C ) +1.60 x 10-19 (0) neutral 4 Proton number • defined as the number of protons in the nucleus. • also called as atomic number, Z. Nucleon number • defined as the total number of neutrons and protons in the nucleus. • also called as atomic mass number, A. Isotopes • defined as the atoms of the same element whose nuclei contain the same number of protons (Z) but different number of neutrons (N). hidrogen deuterium tritium 5 • The atomic nucleus can be represented as A Z X where X = symbol for the element Z = atomic number (number of protons) A = atomic mass number = total number of protons and neutrons Example : 56 26 Fe Iron-56 26 protons 56 – 26 = 30 neutrons A-Z=N 6 Example 26.1 Complete the table below: Element nuclide 1 1 9 4 14 7 16 8 23 11 59 27 31 16 133 55 238 92 H Be N O Na Co S Cs U Number of protons 8 Number of neutrons 8 Number of electrons 8 7 26.2 Binding energy and mass defect (1 1/2 Hours) At the end of this topic, students should be able to: • Define and determine mass defect • Define and determine binding energy, • Identify the average value of binding energy per nucleon of stable nuclei from the graph of binding energy per nucleon against nucleon number. 8 26.2 Binding energy and mass defect Binding energy,E • defined as the energy required to separate a nucleus into its individual protons and neutrons without providing them with kinetic energy. • An alternate interpretation of the binding energy is the energy released (emitted) when the nucleus is formed from its individual nucleons. 9 26.2 Binding energy and mass defect p n n p + p n n p 28.30 MeV To separate a nucleus energy is required p n n p p n n p + 28.30 MeV To form a nucleus energy is released 10 26.2 Binding energy and mass defect Mass defect Δm • defined as the difference between the sum of the masses of individual nucleons that form an atomic nucleus and the mass of the nucleus. Δm Zmp Nmn M A mp : mass of a proton M A mass of a nucleus mn : mass of a neutron Z number of protons N number of neutrons 11 26.2 Binding energy and mass defect • The relationship between the binding energy and mass defect is given by in joule E m c 2 binding energy speed of light mass defect • In nuclear physics, mass is measured in unified atomic mass unit (u). 931.5 MeV 1u c2 E m c2 E m 2 c 12 26.2 Binding energy and mass defect 931.5 MeV 1 eV 1.6 x 10-19 J 1u c2 931.5 x106 1.6 x 10 -19 8 2 3x10 1.656 x 10 -27 kg 931.5MeV - 27 1u 1.66 10 kg 2 c Zm 931.5MeV u EB Zm p Nmn M A c 2 EB p Nmn M A 13 26.2 Binding energy and mass defect • The mean (average) binding energy of a nucleus is callled binding energy per nucleon. Binding energy ( EB ) Binding energy per nucleon Nucleon number( A) E m c 2 mc2 Binding energy per nucleon A 14 Example 26.2 a) Calculate the binding energy of the deuterium. Given 12 H mass 2.013553 u H 11p mass 1.007276 u 1 1 n mass 1.008665 u 1 0 Δm Zm p Nmn M A 11.007276 11.008665 2.013553 0.002388 u 0.002388(1.66 x 10-27 ) 3.96 x 10-30 kg 15 E m c 2 30 E 3.96x10 3x10 8 2 E 3.57x1013 J E 2.23 MeV or 931.5 MeV EB Zm p Nmn M A u EB 11.007276 11.008665 2.013553931.5 MeV EB 2.22 MeV 16 20 b) The binding energy of the neon 10 Ne is 160.647 MeV. Find its atomic mass. Given 1 1 p mass 1.007825 u n mass 1.008665 u 1 0 19.992 u 17 Example 26.3 Calculate the average binding energy per 56 nucleon of the iron-56 26 Fe . Given 56 26 Fe mass 55.93494 u H 11p mass 1.00782 u 1 1 n mass 1.00867 u 1 0 Δm Zm p Nmn M A 261.00782 301.00867 55.93494 0.52848 u 0.52848(1.66 x 10 8.77 x 10-28 kg - 27 ) 18 mc2 Binding energy per nucleon A 8.77 x 10 3 x 10 - 28 8 2 56 1.41 x10 -12 J/nucleon or E = 8.81 MeV/nucleon 19 Exercise Determine the total binding energy and the binding energy per nucleon for the nitrogen -14 14 nucleus 7 N . Given 147 N mass 14.003074 u H 11p mass 1.007825 u 1 1 n mass 1.008665 u 1 0 104.6 MeV,7.47 MeV/nucleon 20 Binding energy per nucleon (MeV/nucleon) Greatest stability Binding energy per nucleon as a function of mass number,A 21 Mass number A • The binding energy per nucleon is a measure of stability of the nucleus. • The greater the binding energy per nucleon, the more stable the nucleus is. From the graph: • For light nuclei, the value of EB/A rises rapidly from 1 MeV/nucleon to 8 MeV/nucleon with increasing mass number A. • For the nuclei with A between 50 and 80, the value of EB/A ranges between 8.0 and 8.9 Mev/nucleon. The nuclei in these range are very stable. 62 Ni has the largest binding • The nuclide 28 energy per nucleon (8.7945 MeV/nucleon). 22 • For nuclei with A > 62, the values of EB/A decreases slowly, indicating that the nucleons are on average, less tightly bound. • For heavy nuclei with A between 200 to 240, the binding energy is between 7.5 and 8.0 MeV/nucleon.These nuclei are unstable and radioactive. Hidrogen with one proton has no binding energy per nucleon. 23