Chapter 11 The Mole
advertisement

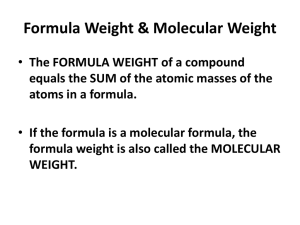
Chapter 11 The Mole Section 11.1 Please have a highlighter and your periodic table out • 3b-Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass exactly of 12 grams. • 3c- Students know one mole equals 6.02 x1023 particles (atoms or molecules) Standard 3 • • • • • • Unit Numerical Value Dozen 12 Pair 2 Ream 500 Gross 144 Mole 6.02 x 1023 Example Eggs in a carton Shoes Sheets of paper Pencils in a box Particles Words used for counting: • 1 dozen is always equal to 12 • 1 mole is always equal to 6.02 ×1023 Never Changes •6.02 ×1023 is also called Avogadro’s number •It was named after this guy: Amedeo Avogadro of Italy Avogardro’s number • The mole is the SI unit used for counting the amount of a substance • 1 mole = 6.02 ×1023 particles Particles can be atoms, ions, molecules, formula units, etc. Other names for a particle • 0.5 moles = 3.01 ×10 23 • 1 mole = 6.02 ×10 23 • 2 moles = 1.20 ×10 24 • 3 moles = 1.80 ×10 24 • 4 moles = 2.41 ×10 24 Multiplying a mole • 602, 000, 000,000,000,000,000,000 particles • 602 SEXTILLION!!!! How big is a mole • The average atomic mass (on the periodic table) tells you how much one mole of an element weighs (it is also called the molar mass). • –Ex. one mole of oxygen atoms weighs 16.00 grams • –Ex. one mole of potassium atoms weighs 39.10 grams 1 mole=atomic mass of each element • Carbon-12 has exactly 6 protons and 6 neutrons • So it has an atomic mass of exactly 12.00 Scientists measured out exactly 12 grams of carbon-12 • The sample had 6.02 ×1023atoms of carbon • They set 6.02 ×1023to be one mole (like 12 is one dozen) How did scientist come up with this number? • • • • • 1. Start with what is given. 2. (given) x -----------3. The units that are given are placed in the denominator 4. Your unknown is placed in the numerator 5. Multiply the top and divide by the bottom Steps for mole caluculations • How many minutes are in 120 seconds? Example of time 1. Determine the number of atoms in 3.00 moles(mol) of Zn? 2. Given 4.00 mole (mol) of AgNO3 determine the number of formula units(FMU). Pg 311 examples • 3. Calculate the number of molecules in 11.5 mol of H2O. • 4a) 5.75 x 1024 atoms Al how many moles? Pg 311 examples continued… • 4b) 3.75 x1024 molecules CO2 How many moles? • 4c) 3.58 x 1023 Formula units ZnCl2 How many moles? • 4d) 2.50 x1020 atoms Fe. How many moles? On your own in your notes needs to be stamped for points Molar Mass and Mole to gram calculation Standard 3.d- Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic masses. • The molar mass of a compound can be calculated solving for the sum of the products of each elements’ mass times the number of atoms present. Molar Mass 17 • EX: Find the molar mass of the following compounds: - P2O5 - C6H12O6 - Fe2(SO4)3 Examples 18 • EX: Find the molar mass of the following compounds: - N2O3 - KC2H3O2 - Ca3(PO4)2 On your own 19 • EX: Perform the following conversions: - Convert 5.0 moles of Na to grams - Convert 12.0 moles of He to grams - Convert 213 grams of NF3 to moles Moles to Grams 20 • PP: Perform the following conversions: - Convert 6.50 moles of O to grams - Convert 25.0 moles of Fe to grams - Convert 0.40 moles of Ne to grams On your own 21