Just as flowers, eggs, and doughnuts are measured in dozens
advertisement

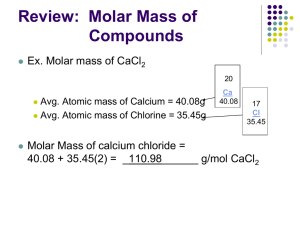
Just as flowers, eggs, and doughnuts are measured in dozens, smaller things, like atoms, molecules, and chemical bonds are measured in moles (abbreviation = mol). Moles allow you to make the following conversions: Think of a mole as a chemist’s dozen. One mole = 6.02 1023 particles 1 mole of any element equals its atomic mass in grams. Just as 1 dozen ostrich eggs are more massive than 1 dozen chicken eggs, 1 mole of Gold atoms is more massive than 1 mole of Sulfur atoms. To find the mass of 1 mole of atoms, just take the atomic mass and change it to grams. Examples: 1 mole of sulfur has a mass of 32.06 g , and 1 mole of Gold is 196.9665 g The number 6.02 1023 is called Avagadro’s Number in honor of Lorenzo Avagadro, who first proposed that equal volumes of gases at equal temperature and pressure would have equal numbers of gas particles. He did not determine this number, but it is named in his honor nonetheless. Moles can be used to measure any “representative unit”, such as atoms, electrons, or eggs. Moles are most commonly used to measure atoms, molecules, ions, and formula units. A formula unit is a group of ions that make up the chemical formula of an ionic compound (such as NaCl, CCl4, and Fe2(SO4)3) Examples: How many molecules in 7.5 moles of NI3? 7.5 mol 6.02 1023 molecules = 4.5 1024 1 mole How many atoms of Iodine in 7.5 moles of NI3? 7.5 mol NI3 6.02 1023 molecules 3 atoms of I = 1.35 1025 1 mole NI3 1 molecule NI3 You can convert moles of a substance to grams of a substance using the general formula: number of moles number of grams in one mole = mass 1 mole Example What is the mass of 3.5 moles of Silicon atoms? 3.5 moles Si 28.0855 g = 98.2993 g 1 mole Si You can convert the mass of a substance into moles of the substance using the general formula: Grams of substance 1 mole = moles of substance number of grams in one mole Example How many moles of Calcium in 260.52 g? 260.52 g 1 mole 40.08 g Ca = 6.5 moles of Calcium One mole of H2O molecules contains 2 moles of Hydrogen (2.0158 g) and 1 mole of Oxygen atoms (15.9994 g), for a combined mass of 18.0153 g. This is called the molar mass of water. The molar mass of a compound is mass in grams of one mole of the compound. Use the following steps to calculate the molar mass of a compound: 1. Determine number of moles of each component element 2. Find the number of grams of each component element 3. Add up the mass of each element to get a combined mass, this is the molar mass of the compound. Example: CCl4 1. This compound contains 1 mole of Carbon atoms, and 4 moles of Chlorine atoms 2. 1 mole of Carbon has a mass of 12.011 g 4 moles of Chlorine has a mass of 4 35.453 g = 141.812 g 3. The molar mass of CCl4 is 12.011 g + 141.812 g = 153.823 g Once you have determined the molar mass of a compound you can use it as you would the atomic weight of an element. Examples: What is the mass of 4.5 moles of CaCl2? (the molar mass of CaCl2 is 110.986 g) 4.5 moles 110.986 g = 499.437 g 1 mole How many molecules of H2O are the in 2000g? (molar mass of water is 18.0153 g) 2000g 1 mole H2O 18.0153g 6.02 1023 molecules = 6.683 1025 molecules 1 mole