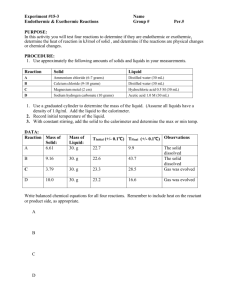
Molar Mass
advertisement

Today You will need a calculator! Go get it Counting Atoms What are some ways that we make it easier to count groups of things? Atoms and molecules are tiny... Counting groups is a pain in the back. 18 grams of water contain 602 000 000 000 000 000 000 000 molecules (more or less...) How can we make this easier? 1 mole = 6.023 x 1023 items We still don’t want to count the things! The mass of one mole is the MOLAR MASS 1 mole of iron = 602 000 000 000 000 000 000 000 atoms = 55.85 grams Posters around the room... Lists of Elements and Compounds, with their Molar Mass I did NOT give any hint as to how I found the molar mass. You know how to look up mass on the periodic table for each atom. You have 5 minutes to look at the posters and figure out what you would need to do. Oxygen O2 32.00 grams/mole Water H2O 18.02 grams/mole Calcium chloride CaCl2 110.98 grams/mole Sodium chloride NaCl 58.44 grams/mole Fluorine gas F2 38.00 grams/mole Sulfur S8 256.56 grams/mole Baking Soda NaHCO3 56.54 grams/mole Potassium permanganate KMnO4 158.04 grams/mole It seemed that...