Special Thanks to…
For sponsorship of LMEF programs:
Supported by an educational grant from Lilly USA, LLC
Did you know that you could be receiving credit for attending
today? For membership information, please visit our website:
www.Lafmeded.org.
Multiple Myeloma 2010
Wael Harb MD
Horizon Oncology Center
Overview
Introduction: epidemiology, clinical
presentation, diagnosis, staging
Autologous stem cell transplantation
Initial approaches to treatment
• Current options
• Novel agents and combinations
Considerations in nontransplantation-eligible patients
Prevention of skeletal complications
What is MM?
Multiple myeloma (MM) is
characterized by the neoplastic
proliferation of a single clone of
plasma cells producing a monoclonal
immunoglobulin.
Plasma Cell
Multiple Myeloma: Incidence
The lifetime risk of getting MM is 1 in
159 (0.63%).
20,180 new cases will be diagnosed
in 2010 (11,170 in men and 9,010 in
women)
10,650 deaths are expected to occur
in 2010 (5,760 in men and 4,890 in
women)
The 5-year relative survival rate for
MM is around 35%
Incidence
MM occurs in all races and all
geographic locations
African Americans and blacks from
Africa is two to three times the risk
in whites
Risk is lower in Asians from Japan
and in Mexicans
Slightly more frequent in men than
in women (1.4:1)
Age
MM is a disease of older adults
The median age at diagnosis is 66
years
Only 10 percent of patients are
younger than 50 years
Only 2 percent of patients are
younger than 40 years
MM: Clinical Presentations
Anemia - 73 percent
Bone pain - 58 percent
Elevated creatinine - 48 percent
Fatigue/generalized weakness - 32
percent
Hypercalcemia- 28 percent
Weight loss - 24 percent, one-half of
whom had lost ≥ 9 kg
Multiple Myeloma = M-CRAB
Monoclonal protein
Calcium
Renal failure
Anemia
Bone pain with lytic lesions
Immunoglobulin
Immunoglobulins
SPEP: Normal
SPEP: M-protein, M-spike
Renal Failure
Cast nephropathy (also called
myeloma kidney) from light chains
Hypercalcemia
Light chain amyloidosis
Drug-induced renal damage
Anemia
Normocytic, normochromic anemiais
present in 73% at diagnosis and in
97%at some time during the course
of the disease
This anemia can be related to:
• Bone marrow replacement
• Kidney damage
• Dilution in the case of a large M-protein
• B12 deficiency in 14%
Rouleaux Formation
Lytic Bone Lesion
MM: PET Scan
Extramedullary Plasmacytoma
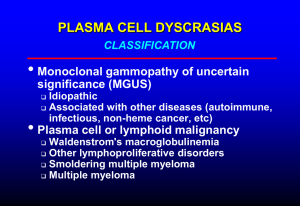
Differential Diagnosis of MM
Monoclonal gammopathy of
undetermined significance (MGUS)
Smoldering multiple myeloma (SMM)
Waldenstrom macroglobulinemia
Solitary plasmacytoma
Primary amyloidosis (AL)
POEMS syndrome
Metastatic carcinoma
Multiple Myeloma
All 3 criteria must be met:
1. Presence of a serum or urinary
monoclonal protein
2. Presence of clonal plasma cells in the
bone marrow or a plasmacytoma
3. Presence of end organ damage felt
related to the plasma cell dyscrasia,
such as:
•
•
•
•
Increased calcium concentration
Lytic bone lesions
Anemia
Renal failure
Smoldering Multiple Myeloma
SMM, Asymptomatic
Both criteria must be met:
Serum monoclonal protein ≥3 g/dL
and/or bone marrow plasma cells
≥10 percent
No end organ damage related to
plasma cell dyscrasia
Monoclonal Gammopathy of
Undetermined Significance
(MGUS)
All 3 criteria must be met:
Serum monoclonal protein <3 g/dL
Bone marrow plasma cells <10
percent
No end organ damage related to
plasma cell dyscrasia or a related B
cell lymphoproliferative disorder
POEMS Syndrome
Osteosclerotic myeloma
• Polyneuropathy
• Organomegaly
• Endocrinopathy
• Monoclonal protein
• Skin changes
MM: Evaluation
CBC and differential,peripheral blood
smear
Chemistry:serum calcium, creatinine,
albumin, LDH , beta-2 microglobulin, and
C-reactive protein
Serum protein electrophoresis (SPEP) + IF
Quantification of immunoglobulins
Urinalysis and a 24-hour urine collection
for electrophoresis (UPEP) + IF
Serum free monoclonal light chain (FLC)
MM Evaluation
Serum viscosity should be measured if
the M-protein concentration is high
Bone marrow aspiration and biopsy
with immunophenotyping, conventional
cytogenetics, and fluorescence in situ
hybridization (FISH)
Metastatic bone survey with plain
radiographs including the humeri and
femoral bones should be performed in
all patients.
Bone Marrow
Cytogenenetics, Interphase
FISH
Poor prognosis (median survival 25
months): t(4;14)(p16;q32),
t(14;16)(q32;q23), and
-17p13
Intermediate prognosis (median
survival 42 months): -13q14
Good prognosis (median survival 50
months): all others
Staging for MM
International staging system (ISS)
Stage I — B2M <3.5 mg/L and
serum albumin ≥3.5 g/dL
Stage II — neither stage I nor
stage III
Stage III — B2M ≥5.5 mg/L
Median overall survival for patients
with ISS stages I, II, and III are
62, 44, and 29 months
MM: Treatment Decisions
Indications for treatment
Risk stratification
Eligibility for stem cell
transplantation
Smoldering (asymptomatic)
myeloma
Deferral of chemotherapy until
progression to symptomatic disease
Follow these patients closely, every 3
to 4 months, with serum protein
electrophoresis, complete blood
count, serum creatinine, and serum
calcium
Metastatic bone survey should be
considered annually because
asymptomatic bone lesions may
develop
MM: Indications for Treatment
Anemia (hemoglobin <10 g/dL or 2
g/dL below normal)
Hypercalcemia (serum calcium >11.5
mg/dL)
Renal insufficiency (serum
creatinine>2 mg/dL)
Lytic bone lesions or severe
osteopenia
Extramedullary plasmacytoma
MM: RISK STRATIFICATION
FISH for detection of t(4;14), t(14;16),
and del17p13
Conventional cytogenetics (karyotyping)
for detection of del 13 or hypodiploidy
The presence of any of the above markers
defines high risk myeloma, which
encompasses the 25 percent of MM
patients who have a median survival of
approximately two years or less despite
standard treatment
Current Frontline Options
Conventional chemotherapy
• Survival ≤ 3 yrs
Transplantation
• Prolongs survival 4-5 yrs
Novel agents targeting stromal
interactions and associated signaling
pathways have shown promise
Chng WJ, et al. Cancer Control. 2005;12:91-104.
MM: INITIAL THERAPY
The initial therapy of patients with
symptomatic myeloma varies
depending on whether patients are
eligible or not to pursue autologous
hematopoietic cell transplantation
Initial Approach to Treatment of MM
Clearly not transplantation
candidate based on age, performance
score, and comorbidity
Potential transplantation
candidate
MPT, MPV, Len/dex
or clinical trial*
Nonalkylator-based
induction x 4 cycles
*Thal/dex or dex are additional options
especially if immediate response is needed.
Stem cell harvest
DETERMINING TRANSPLANT
ELIGIBILITY
Autologous hematopoietic cell
transplantation (HCT) results in superior
event-free and overall survival rates when
compared with combination chemotherapy
All patients should be evaluated at
diagnosis for transplant eligibility so that
the risks and benefits of autologous HCT
can be reviewed with those eligible
A minority of patients will be eligible for
allogeneic HCT, but the value of allogeneic
approaches in myeloma remain
investigational
NOT Eligible for Autologous
HCT
Age >77 years
Direct bilirubin>2.0 mg/dL (34.2
µmol/liter)
Serum creatinine>2.5 mg/dL (221
µmol/liter) unless on chronic stable
dialysis
Eastern Cooperative Oncology Group
(ECOG) performance status 3 or 4
unless due to bone pain
New York Heart Association
functional status Class III or IV
Transplantation vs Conventional
Chemotherapy
100
100
54
75
High dose
Survival (%)
OS (%)
75
50
Conventional dose
25
Intensive therapy
42
50
Standard therapy
25
P = .03 by Wilcoxon test
P = .04 by log-rank test
0
0
0
15
30
Mos
45
60
0
20
40
Mos
Attal M, et al. N Engl J Med. 1996;335:91-97. Child JA, et al. N Engl J Med. 2003;348:1875-1883.
60
80
Autologous Stem Cell
Transplantation
Mel 200 mg/m2 standard conditioning regimen
Sufficient performance score, and adequate liver,
pulmonary, cardiac function needed
Higher PR and CR rates than conventional
chemotherapy
Higher OS and EFS than conventional Rx
Advanced age and impaired renal function are, by
themselves, not contraindications
Attal M, et al. N Engl J Med. 1996;335:91-97. NCCN Practice Guidelines. Myeloma. V.3.2010.
Stem Cell Transplantation
Key issues
Efficacy compared with conventional chemotherapy
Timing: early vs delayed
Single vs tandem
Role of allogeneic and miniallogeneic transplantations
Maintenance post-SCT
Novel Frontline Options
Immunomodulatory drugs (IMiDs)
• Thalidomide
• Lenalidomide
Proteasome inhibitors
• Bortezomib
• Carfilzomib
Proposed Mechanism of Action for
Multiple Myeloma Therapies
Kyle RA, et al. N Engl J Med. 2004;351:1860-1873.
Copyright ©2004. Massachusetts Medical Society. All rights reserved.
Thalidomide:
Proposed Mechanism of Action
Proposed mechanisms
• Inhibition of TNF-
• Suppression of angiogenesis
• Increase in cell-mediated cytotoxic
effects
• Modulation of adhesion molecule
expression
Kyle RA, et al. N Engl J Med. 2004;351:1860-1873. Rajkumar SV, et al. Leukemia. 2003;17:775-779. D’Amato RJ, et
al.Proc Natl Acad Sci U S A. 1994;91:4082-4085.
Lenalidomide
Immunomodulatory derivative of
thalidomide
More potent than thalidomide in preclinical
models
• Dose-dependent decrease in TNF-α and
interleukin-6
• Directly induces apoptosis, G1 growth arrest
• Enhances activity of dexamethasone
More favorable toxicity profile than
thalidomide
Richardson P, et al. Blood. 2003;100:3063. Hideshima T, et al. Blood. 2000;96:2943-2950.
Bortezomib:
A Reversible Proteasome Inhibitor
Cross section of b ring
b1
O
N
N
N
H
H
N
OH
B
OH
PostGlutamyl
Site
Tryptic
Site
b7
b3
Bortezomib
O
b6
b4
b5
Adams J, et al. Invest New Drugs. 2000;18:109-121.
Adams J, et al. Bioorg Med Chem Lett. 1998;8:333-338.
b2
Chymotryptic
Site
Initial Approach to Treatment of MM
Clearly not a
transplantation candidate
Potential transplantation
candidate
MPT, MPV, Len/dex
or clinical trial*
Nonalkylator-based
induction
Stem cell harvest
Melphalan/Prednisone/Thalidomide
Melphalan/
Prednisone
(n = 164)
Melphalan/
Prednisone/
Thalidomide
(n = 167)
HR (95% CI)
P Value
Median PFS, mos
14.5
21.8
0.63 (0.48-0.81)
.0004
No. of events
125
111
--
--
Median OS, mos
47.6
45.0
1.04 (0.76-1.44)
.79
No. of events
70
77
--
--
Outcome
Palumbo A, et al. Blood. 2008;112:3107-3114.
Len + High or Low-Dose Dex in
Newly Diagnosed Myeloma (E4A03)
Courses repeat every 28 days ≤ 1 yr in absence of
PD or unacceptable toxicity
Untreated,
symptomatic
myeloma,
no age cutoff
Lenalidomide 25 mg/day PO on
Days 1-21 +
High-dose Dex 40 mg/day PO
on Days 1-4, 9-12, 17-20
(n = 223)
Lenalidomide 25 mg/day PO on
Days 1-21 +
Low-dose Dex 40 mg/day PO
on Days 1, 8, 15, 22
(n = 222)
Total Dex dose
per cycle:
480 mg
Total Dex dose
per cycle:
160 mg
Rajkumar SV, et al. ASCO 2008. Abstract 8504. Rajkumar SV, et al. Lancet Oncol. 2010;11:29-37
Len + High or Low-Dose Dex
(E4A03): Response
High Dose
Low Dose
P Value
Overall response at 4 cycles, %
79
68
.008
≥ VGPR within 4 cycles, %
42
24
< .0001
Best overall response, %
81
70
.009
≥ VGPR, %
50
40
.040
CR (IF-), %
13
10
--
1-yr OS
87
96
.0002
2-yr OS
75
87
--
OS, %
3-yr OS rates converged (P = .467) with all pts crossed over to low
dose
Successful stem cell harvesting in 97.6% (n = 167)
3-yr OS for high dose or low dose followed by SCT: 92%
Rajkumar SV, et al. Lancet Oncol. 2010;11:29-37.
Len + High or Low-Dose Dex
(E4A03): Adverse Events
Toxicity Grade ≥ 3, %
High Dose
Low Dose
P Value
Toxicity (any) during first 4
mos, grade ≥ 3
52
35
.0001
Nonhematologic (any),
grade ≥ 3
65
48
.0002
Death (early < 4 mos)
5
0.50
.003
DVT/PE
26
12
.0003
Infection/pneumonia
16
9
.04
Neutropenia
12
20
.02
Rajkumar SV, et al. Lancet Oncol. 2010;11:29-37.
Lenalidomide Dosing for MM and
Impaired Renal Function
Renal Impairment (CrCl)
Moderate (30 to < 60 mL/min)
Severe (< 30 mL/min, not requiring
dialysis)
ESRD (< 30 mL/min, requiring dialysis)
Lenalidomide [package insert].
Lenalidomide Dosage
10 mg QD
15 mg Q 48 hrs
5 mg QD
On dialysis days,
administer following
dialysis
Peripheral Neuropathy Following
Bortezomib Therapy in Advanced
MM
Peripheral neuropathy was reported in
90/256 (35%) patients with MM
treated with bortezomib in phase II
trials
80% of patients entered these trials with preexisting
peripheral neuropathy
3% patients without vs 16% with baseline peripheral
neuropathy developed grade 3 peripheral neuropathy
Richardson PG, et al. ASH 2003. Abstract 512.
Frontline Therapy in Elderly MM
Patients
For elderly patients or those who are not
suitable candidates for transplantation, MP
has been a standard treatment
• ORR: 60%
• Long-term CR: < 5%
Trials with MP-based combinations reported
improved response rates and time to
progression
• MPT
NCCN Practice Guidelines. Myeloma. V.3.2010.
• VMP
Conclusions
In elderly patients, the addition of novel
agents to standard MP has provided
improved response rates
• MP alone (ORR: 50%; CR: 5%)
• MPR (50% to 95% reduction in myeloma
protein in 55.6%)
• VMP (ORR: 86%)
Care should be taken with IMiD-based
therapy to include aspirin prophylaxis for
DVT/PE
Care should be taken with bortezomibbased regimens to include herpes zoster
prophylaxis
MM & Skeletal Complications
~ 80% of patients
with multiple myeloma
will have evidence of
skeletal involvement
on skeletal survey
• Vertebrae: 65%
• Ribs: 45%
• Skull: 40%
• Shoulders: 40%
• Pelvis: 30%
• Long bones: 25%
Dimopoulos M, et al. Leukemia. 2009:1-12.
The Central Role of the
Osteoclast in Osteolytic Bone
Destruction
Tumor cells
Osteoclast differentiation
Growth
factors
Direct effects on
osteoclast differentiation
Active
osteoclast
Osteolysis
Bone loss
Adapted from Roodman GD. N Engl J Med. 2004;350:1655-1664.
Mechanism of Bisphosphonate
Inhibition of Osteoclast Activity
Bisphosphonates
inhibit osteoclast
activity, and promote
osteoclast apoptosis[1]
X
Bisphosphonates may modulate
signaling from osteoblasts
to osteoclasts
Increased OPG production[2]
Decreased RANKL expression[3]
New bone
Bone
Bisphosphonates
are released locally
during bone resorption[1]
Bisphosphonates are
concentrated under
osteoclasts[1]
1. Reszka AA, et al. Curr Rheumatol Rep. 2003;5:65-74. 2. Viereck V, et al. Biochem Biophys Res
Commun. 2002;291:680-686. 3. Pan B, et al. J Bone Miner Res. 2004;19:147-154.
Recommended Doses and
Infusion Times
Drug
Dose/Infusion
Time
Interval
Estimated CrCl > 60 mL/min
Pamidronate
Zoledronic acid
90 mg over 2-3 hrs
4 mg over 15 mins
3-4 wks
3-4 wks
Estimated CrCl 30 to < 60 mL/min
Pamidronate
Zoledronic acid
90 mg over 2-3
hrs*
Reduced dosage†
3-4 wks
3-4 wks
Estimated CrCl < 30 mL/min
Pamidronate
*Consider
dose reduction
Zoledronic
acid.
90 mg over 4-6
3-4 wks
hrs*
†3.5mg (CrCl 50-60 mL/min); 3.3 mg (CrCl 40-49 mL/min); 3.0 mg (CrCl 30-39 mL/min).
Not recommended
Kyle R, et al. J Clin Oncol. 2007;25:2464-2472.
Bisphosphonates and
Osteonecrosis
Papapetrou PD. Hormones (Athens). 2009;8:96-110.
Uncommon
complication causing
avascular necrosis of
maxilla
or mandible
Suspect with tooth or
jaw pain or exposed
bone
May be related to
duration of therapy
True incidence
unknown
Normal RANKL/OPG
OPG
RANKL
Prevents
Promotes
Osteoclastic Activity
Hofbauer LC, et al. JAMA. 2004;292:490-495.
The RANK/RANKL/OPG
Pathway in Osteolytic Bone
Disease
OPG
Prevents
RANKL
Promotes
Increased osteoclastic
activity and
decreased OPG
Adapted from Roodman GD. N Engl J Med. 2004;350:1655-1664.
Denosumab: Inhibiting RANK in
Bone Disease
High affinity human monoclonal
antibody that binds RANKL
Administered via SC injection
Specific: does not bind to TNF-α,
TNF-β, TRAIL, or CD40L
Inhibits formation and activation of
osteoclasts
Multiple Myeloma = M-CRAB
Monoclonal protein
Calcium
Renal failure
Anemia
Bone pain with lytic lesions
Thank you