Equipment Management C.D.D.F.T.
advertisement

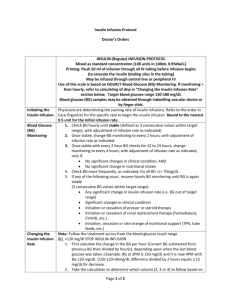
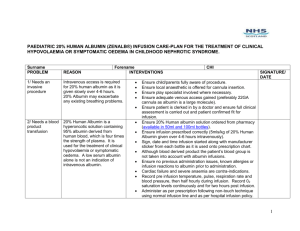
Equipment Management County Durham & Darlington Foundation Trust (C.D.D.F.T.) C.D.D.F.T. Organisational Statistics • Serves a population of: 500,000 • Number of in-patients treated per year: 83,314 • Employs 6000 staff • Annual Income: £330 million C.D.D.F.T. Background • Medical Devices Policy - HSJ Award 2005 …also related policies – Infusion Device Policy and Training & Competency Policy for Medical Devices. • Established Network of Equipment Controllers • Medical Devices Group – review of MHRA alerts with Risk Management, review of Trust incident reports with Patient Safety Managers. • Clinical Engineering – Team Leaders monthly review of medical devices related incidents. Background • 2003/2004 early stages of amalgamation of other sites into what is now County Durham & Darlington Foundation Trust (C.D.D.F.T.) • Various types of equipment were in use across the developing Trust prompting the introduction of a standardisation programme. Background • Decision made to centralise some equipment, initially on one Trust site - Darlington • Prior to this, infusion devices and pressure relief systems were with users, stored on wards. • An audit of infusion device usage was carried out • Results ‘mirrored’ the NPSA audit – Pilot Study (2004) - that at any one time there was 65% non-utilisation • Pressure relief systems were purchased based on utilisation of rental equipment. Central Equipment Loan Library (C.E.L.L.) • In October 2004 the Central Equipment Loan Library (C.E.L.L.) was opened at Darlington • Pressure relief systems and infusion devices chosen • The service included visual checks of the equipment, cleaning/decontamination and tracking of individual patient use via an electronic database, providing audit trails. • C.E.L.L. based in Clinical Engineering Dept making it easy for equipment to be located for Planned preventative maintenance (P.P.M.) and relevant repairs/service to be carried out Equipment Tracking C.E.L.L. C.E.L.L. Pressure Relief Mattress Systems (P.R.S.) • PRS are requested from C.E.L.L. by ward staff following patient evaluation and delivered by C.E.L.L. staff • Patient details and ward location are recorded against the PRS asset reference. The individual base cover and top cover numbers are also recorded – providing audit trail. Pressure Relief Mattress Systems (P.R.S.) • In 2008 the provision of P.R.S was expanded to another Trust site twelve miles away by operating a satellite service from the Darlington site. • Deliveries and collections are made at least three times per week. • P.R.S. are distributed via the Linen Bank, patient details sent to Darlington to be added to the data base. Pressure Relief Mattress Systems (P.R.S.) • 2009 – another C.E.L.L. opened at the University Hospital of North Durham (UHND), providing the same service. • 2010 – further satellite service for P.R.S. from UHND opened providing equipment to Chester le Street Hospital and Shotley Bridge Hospital. • Training is provided by both the Medical Devices Nurses and the Tissue Viability Team in conjunction with the manufacturer. • Mandatory Training changed with the addition of a Medical Devices workshop. P.R.S. Inventory • January 2005 – ninety five pressure relief mattresses across seven different products • June 2011 – three hundred and forty mattresses across four different products plus seat cushions Utilisation • P.R.S. utilisation for first full financial year 2005 -2006 18,796 bed days • P.R.S. utilisation for 2010 – 2011 financial year 67,211 bed days Pressure Ulcer Prevalence Prevalence Trend 2003 - 2010 7 6 5 Overall 4 Hospital Acquired 3 Admitted w ith 2 1 0 2003 2004 2005 2006 2007 2008 2009 2010 High Risk % WLS>20 High/Very High Risk % WLS>20 60 50 40 30 Risk % 20 10 0 2006 2007 2008 2010 Tissue Viability • Development of Tissue Viability team in 2004 & 2006 • Replacement of all Kings fund bed frames for electronic profiling bed frames • Replacement of standard hospital mattress for pressure reducing foams as standard for all patients • Annual audit of all surfaces ie. Mattresses, chairs, trolleys etc • From 2011 monthly auditing of all mattresses Falls Harm Rates/Morbidity • Global Trigger Tool and Mortality 3 x 2 used every month, • Minimum of 50 case note reviews – 3.2% for 2010/2011 • Any equipment issues identified from audits are actioned. Infusion Devices • The infusion device standardisation programme was completed October 2010 • The general areas all use the same model of infusion devices that are configured the same Trust wide. • Specialist areas also use the same models which are also configured the same Trust wide. • Competency based training is provided monthly on a rotational site basis Infusion Devices • At DMH and UHND, infusion devices for the general areas are accessed by requesting them from CELL. • Patient details and ward location are recorded on the electronic database against the device asset number, providing an audit trail. • Devices are easily made available for P.P.M due to proximity of Clinical Engineering Dept. • Although no metrics gathered, anecdotally harm & incidents have reduced The Way Ahead • Since August 2010 Clinical Engineering services have been working in the PCT with Medical Devices Nurse (Community) to review and develop an up to date inventory of equipment for P.P.M • April 2011 PCT integrated into C.D.D.F.T. • Medical Devices Nurse (Acute) and Medical Devices Nurse (Community) now working together in the same Trust. The Way Ahead Several work streams have been identified and are being developed including; •Using the Community equipment inventory to develop a Training Needs Analysis (T.N.A.) for Community Division and incorporating it into the already established T.N.A. for the Acute Divisions •Identifying and including Equipment Controllers (Link staff) for medical devices from the Community sector and including them in the already established Equipment Controller list facilities and training. •Identifying Community sector representatives to established Medical Devices Group •Standardisation of equipment to ensure smooth transition of patients between the Community & Acute settings. There is ongoing work with Palliative Care Team to reduce readmissions through availability of equipment and the care pathways. •Operating satellite services for P.R.S to Community Hospitals The Way Ahead • Introduction of ‘Guardrails’ – a patient safety dosing system, to infusion devices • Introduction of the McKinley T34 ambulatory pump into the Acute Divisions – already established in the Community Divisions …Other Teams Working to Reduce Harm • Clinical Procurement Standardisation Group – 150 commonly used consumables • Latex Reduction Group • Tracheostomy Care – In response to an alert from a coroner about patients with tracheotomy a multi-disciplinary group was established to review the key learning points. Any gaps were then actioned. • An insulin device guide has been produced UHND after gaps were identified in clinical staff’s awareness and knowledge of the multiple devices currently available. • The development of the PYJown – making it easier for patients to dress, but also maintaining dignity and allowing access for I.V extension lines. Clinical Engineering Team