New Oral Anticoagulants: A
Review
Babak Moini, MD
Veterans Affairs Hospital
Noon Lecture Series
Acknowledgment:
Some of the slides were borrowed from Amanda Miller
Phar.D.
Case1
68 male with hx of DM, CHF and prior ischemic CVA
admitted for new afib. He has a hx of non-compliance.
CHADs2: 4.
Which anticoagulant to send him home with?
Oral Anticoagulants Available in
US
Coumadin
warfarin
Pradaxa®
dabigatran
Xarelto®
rivaroxaban
Eliquis®
apixaban
1954
2010
2011
2012
Mechanism
of Action
Mechanism
of Action
Medication
Coumadin (warfarin)
Vitamin K Antagonist
Pradaxa (dabigatran) Direct Thrombin Inhibitor
Xarelto (rivaroxaban) Factor Xa Inhibitor
Eliquis (apixaban)
Factor Xa Inhibitor
rivaroxaban
apixaban
dabigatran
http://www.healio.com/~/media/Images/News/Online/Orthopedics/2009/12_December/01/79_fig_400_307_
57368.gif
Pharmacology:
Coumadin
Dabigatran
Rivaroxaban
Apixaban
Bioavailability
100%
60-100%
50%
Protein bound
99%
90-95%
80-85%
Metabolism
CYP
Conjugation
CYP
CYP
Half Life
40hrs
12-17hrs
5-9hrs
12hrs
Onset of
action
72hrs
1-2hrs
2-4hrs
2-4hrs
Elimination
Liver
Renal
Renal
Renal
Indications:
Coumadin
Dabigatrn
Afib
(RELY)
DVT/PE
Not yet.
(RECOVER)
Post TKA/THA
DVT
prophylaxis
+
Rivaroxaban
(ROCKETAF)
Apixaban
(Aristotle)
+
(AVERROES)
(EINSTEIN)
(RECORD
1-3)
+ (ADVANCE)
Not yet approved: Rivaroxaban for prophylaxis of DVT in medically ill patients
(MAGELLAN). Rivaroxaban vs Enoxaparin. NI < 30 days, superior at 35 days.
Usual Dosing (A fib)
Warfarin
• Once daily, titrate to INR 2-3
Dabigatran
• 150 mg BID
• 75 mg BID (CrCl 15-30 ml/min)
Rivaroxaban
• 20 mg daily
• 15 mg daily (CrCl 15-50 ml/min)
Apixaban
• 5 mg BID
• 2.5 mg BID if any 2 of the following: age
> 80, wt < 60kg, SCr > 1.5
Usual Dosing (VTE)
Only FDA-approved agent = rivaroxaban
VTE Prophylaxis (knee/hip surgery)
10mg once daily (up to 35 days)
No renal dose (CrCl < 30 ml/min avoid)
VTE Treatment:
15 mg BID x 3 weeks then 20mg daily
No renal dose (CrCl < 30 ml/min avoid)
Perioperative Recommendations
Dabigatran
• Hold 1-2 days before procedure
• CrCl < 50 hold 3-5 days
• Low bleed risk hold 1 day
Rivaroxaban
• CrCl < 30/ low risk hold 2 days
• High bleed risk hold 2 days
• CrCl < 30/ high risk hold 4 days
Apixaban
• Low bleed risk hold x 1 day
• High bleed risk hold x 2 days
Dabigatran PI, Blood 2012;119:3016-23
Major Side Effects:
Bleeding: varied definition in each study.
GI
ICH
Major (drop in Hgb by 2, life threatening).
Dabigatran:
Pills are made in acidic content, hence has 20% rate of
GI side effects.
? Observed increase risk of GI bleeding.
Monitoring Levels:
Coumadin: INR
New Oral anticoagulants: no standardized studies. No
accurate quantitative measures.
Dabigatran: ECT, Thrombin clotting time
Rivaroxaban: special anti-Xa activity
Abixaban special anti-Xa activity
Drug-Drug Interactions:
No where as severe as with Warfarin.
Dabigatran: P-glycoprotein, pro-drug.
Needs acidic environment, avoid co-administration with
PPI.
Rivaroxaban: CYP-450 and P-glycoprotein.
Caution with dual inhibitors (Ketoconazole, Itroconazole,
Clarithromycin).
No dose adjustments needed.
Abixaban: CYP3A4 and P-glycoprotein.
Decrease dose to 2.5mg bid in dual inhibitors.
Switching To/From Warfarin
Medication
Recommendations for Conversion
Stop warfarin, initiate dabigatran when INR < 2
Dabigatran
Initiate warfarin 3 days before D/C dabigatran
Stop warfarin, initiate rivaroxaban when INR < 2-3
Rivaroxaban
Initiate warfarin with bridging 24 hours after D/C
rivaroxaban
Stop warfarin, initiate apixaban when INR < 2
Apixaban
Initiate warfarin with bridging when next apixaban
dose is due.
Treatment of Bleeding:
No evidence based guidelines.
Remember that unlike Coumadin, the new OAC will
continuously bind to factor Xa or thrombin, hence
making FFP less useful.
Current available Rx for life threatening active bleeding:
based on case reports.
Factor VII
PCC: 3 and 4 factor concentrates.
HD: only for Dabigatran. Large volume of distribution.
Charcoal
Gonsalves Et al. Mayo Clinic Proc. 5-2013
Trials vs Warfarin for A Fib
RE-LY
DAB vs WAR
ROCKET-AF
RIV vs WAR
ARISTOTLE
APIX vs WAR
Dabigatran
Rivaroxaban
Apixaban
Open-label,
blind outcomes,
noninferiority
Double-blind,
noninferiority
Double-blind,
noninferiority
n = 18,113
n = 14,264
n = 18,201
Randomization
D 150mg BID
D 110mg BID
W (INR 2-3)
R 20mg daily*
W (INR 2-3)
A 5mg BID*
W (INR 2-3)
Inclusion
Criteria
Nonvalvular AF
with increased
stroke risk
Nonvalvular AF
with prior stroke
or >2 risk factors
Nonvalvular AF
with >1 risk factor
for stroke
Exclusion
CrCl < 30
CrCl < 30
CrCl < 25
Comparator
Design
Sample size
* Dose reductions for renal impairment
Trials vs Warfarin for A fib
RE-LY
DAB vs WAR
ROCKET-AF
RIV vs WAR
ARISTOTLE
APIX vs WAR
Average age (yrs)
71
73
70
Mean CHADS2
2.1
3.5
2.1
0-1
32%
0%
34%
2
36%
13%
36%
3-6
32%
87%
30%
Prior TIA/stroke
20%
55%
19%
TTR (INR @ goal)
64%
55%
62%
Median follow-up
2 yrs
1.9 yrs
1.8 yrs
Primary endpoint
Stroke (ischemic, hemorrhagic) + systemic embolism
Major Findings:
RELY
Dabigatran 110mg NI to Warfarin (1.53% vs 1.69%).
Dabigatran 150mg superior to Warfarin ONLY if compared
with sub-optimal INR subgroup (1.11 % vs 1.69%).
Major bleeding less with 110mg (2.71 vs 3.11%).
ROCKET-AF
Rivaroxaban NI to Warfarin (2.1% vs 2.4%)
Less ICH or fatal bleeding (0.4% vs 0.8% )
ARISTOTLE:
Abixaban Superior to Warfarin (1.27% vs 1.6% )
Less Major bleeding (1.4% vs 2.1% )
Key Safety Endpoints (% per
year)
RE-LY
ROCKET AF
ARISTOTLE
D110
D150
WAR
RIV
WAR
APIX
WAR
1o bleeding
endpoint*
2.71
3.11
3.36
14.9
14.5
2.13
3.09
Major bleed
2.71
3.11
3.36
5.55
5.42
2.13
3.09
GI bleeding
1.12
1.51
1.02
3.2
2.2
0.76
0.86
Intracranial
hemorrhage
0.23
0.3
0.74
0.5
0.7
0.33
0.8
*: Primary safety endpoint:
o RE-LY major hemorrhage
o ROCKET-AF major + non-major clinically relevant bleeding
o ARISTOTLE ISTH (Int Soc Thromosis & Hemostasis) major
bleeding
Figure 3 Forest plot for (A) major bleeding, (B) intracranial bleeding, and (C) gastrointestinal bleeding, new oral anticoagulants
(NOA) versus warfarin in patients with AF.
http://dx.doi.org/10.1016/j.amjcard.2012.03.049
Quick Review of EvidenceBased Medicine:
I
A: Systemic review of multiple RCTs / multiple RTCs
B: High quality single RTC
II:
A: Systemic review of cohort studies
B: High quality cohort studie(s)
III:
Systemic review of Case/Control studies / Case Control studies
IV
Case reports
IV
Expert opinion
Anticoagulation Recommendations (AF)
Risk/CHADS2
CHEST 2012
AHA/ASA
No therapy > antithrombotic therapy (2B)
Low Risk
CHADS2 = 0
Intermediate
CHADS2 = 1
Aspirin (1A)
Aspirin (75-325mg) > OAC (2B) or aspirin + clopidogrel
(2B)
OAC > no therapy (1B)
Warfarin (1A)
OAC > aspirin (2B) or aspirin + clopidogrel (2B)
Aspirin, if patient
preference (1A)
OAC unsuitable or pt refuses: aspirin + clopidogrel over
aspirin monotherapy (2B)
OAC > no therapy (1A)
Warfarin (1A)
Dabigatran (1B)
Rivaroxaban (2A)
Apixaban (1B)
OAC > aspirin (1B) or aspirin + clopidogrel (1B)
High Risk
CHADS2 > 2
OAC unsuitable or pt refuses: aspirin + clopidogrel over
aspirin monotherapy (1B)
Dabigatran 150mg BID > warfarin (2B)
OAC = oral anticoagulation
Chest 2012; 141:e531Se575S
Stroke 2012;43: 3442-3453
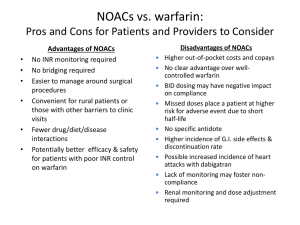
New OAC:
Pros:
Easy administration
Immediate effect
Much less food and drug
interactions
One dose fits all
The names sound so much
cooler than WARFARIN.
Cons:
Expensive
Inability to monitor
compliance
Short duration: loss of effect
with a single missed dose
No safe/reliable antidotes
? Bleeding. Observational
bias vs real difference.
Renal dosing
Take home message:
Coumadin still remains the drug of choice for many patients
due to cost, past experience and known side effects.
Many new OAC are in the pipeline, expect a barrage of
pharma bombardments, must remain objective as many of
the studies have different inclusion/exclusion criteria,
definition of end points and side effects.
Each patient may benefit from a different type of OAC based
on comorbidities and drug side effect profile.
Watch out for recall bias with the new OAC among your own
colleagues.
Patient compliance is a major factor: remember with the new
OAC one missed dose means a lot!
The End!